Translate this page into:
Acute Bilateral Supranuclear Vertical Gaze Palsy: Vertical One-and-a-one Syndrome – Report of Three Cases
Address for correspondence: Dr. Rohan R. Mahale, Department of Neurology, M.S Ramaiah Medical College and Hospital, Bengaluru - 560 054, Karnataka, India. E-mail: rohanmahale83@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), interstitial nucleus of Cajal (INC), and posterior commissure (PC), located in the tegmentum of the midbrain, are the brain stem centers for vertical eye movement.[1] Unilateral damage to the thalamo-mesencephalic junction is caused by stroke in the territory of the thalamo-subthalamic paramedian artery. The resulting damage causes vertical gaze abnormalities as they interrupt the descending fibers controlling the vertical gaze.[2] Vertical gaze abnormalities that are reported due to damage to unilateral thalamo-mesencephalic junction include vertical one-and-a-half syndrome, contralesional monocular elevation palsy, coexistence of vertical and horizontal one-and-a-half syndrome, and vertical half-and-a-half syndrome.[3456] Rarely, paramedian thalamic infarction can also present as vertical gaze palsies. Hereby, we describe three patients who presented with unique vertical gaze disorder in the form of acute bilateral conjugate supranuclear vertical gaze palsy due to infarct in the unilateral thalamo-mesencephalic junction and isolated medial thalamus.
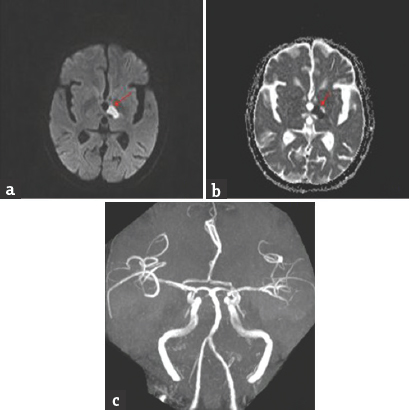
Case 1: A 42-year-old male presented with a history of gait unsteadiness of 2-day duration. He used to sway on to his left side while walking. Gait unsteadiness was associated with blurring of vision on looking near objects. There was no upper limb incoordination or speech difficulty. He did not have headache, vomiting, or sensory disturbances. He was diabetic on medications with good glycemic control. He had marfanoid habitus. Systemic examination was unremarkable. His pulse rate was 86/min and blood pressure was 132/86 mmHg. He was conscious and oriented. Speech was normal. Fundus examination was normal. Pupils were equal and reactive. Oculomotor abnormalities were noted in the form of restricted up and down gaze in both eyes. Vertical vestibulo-ocular reflex was preserved. Convergence was restricted on the left side. Horizontal gaze was preserved in both eyes [Figure 1]. Rest of the cranial nerves was normal. Motor examination revealed normal tone, power, and reflexes in all limbs. Sensory examination was normal. Finger–nose coordination was normal, but left knee–heel coordination was impaired. Tandem gait was impaired with a sway to the left side. Plantar responses were flexor. Complete hemogram, renal, hepatic, and thyroid functions were normal. Glycated hemoglobin was 7.6%. Brain magnetic resonance imaging (MRI) showed acute infarct in the right anteromedial thalamus and right rostral ventral paramedian midbrain. MR angiography of intracranial arteries showed thinning of the right posterior cerebral artery (PCA) [Figure 2]. Carotid and vertebral arteries Doppler were normal. Two-dimensional (2D) transthoracic echocardiography showed mild aortic regurgitation. Serum homocysteine level was high. Serological testing for human immunodeficiency virus, hepatitis B surface antigen, and venereal disease research laboratory was negative. He was treated with antiplatelets, statin and gait, and balance exercises. At 1-month follow-up, there was an improvement in gait unsteadiness with mild improvement in vertical gaze.

- Brain magnetic resonance imaging-diffusion-weighted imaging axial view (a and b) bright signal change in the right paramedian midbrain and anteromedial thalamus (red arrow); apparent diffusion coefficient axial view (d and e) corresponding dark signal change (red arrow); magnetic resonance angiography of intracranial arteries shows thinning of right posterior cerebral artery (red arrow)(c)
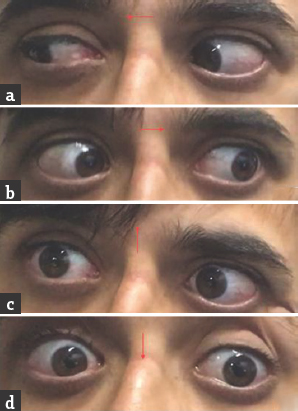
- (a and b) Normal horizontal eye movements; (c and d) restricted vertical eye movements
Case 2: A 54-year-old female presented with a history of double vision of 1-day duration. It was acute in onset with images appearing up and down when both eyes were open. She did not have headache, vomiting, motor weakness, sensory disturbances, or limb incoordination. She was hypertensive on medications. General physical and systemic examination was normal. Her pulse rate was 84/min and blood pressure was 154/96 mmHg. She was conscious and oriented. Speech was normal. Fundus examination was normal. Pupils were equal and reactive. Diplopia was present in upgaze. Oculomotor abnormalities were noted in the form of restricted up and down gaze in both eyes. Vertical vestibulo-ocular reflex was preserved. Convergence was restricted on both sides. Horizontal gaze was preserved in both eyes [Figure 3]. Rest of the cranial nerves was normal. Motor examination revealed normal tone, power, and reflexes in all limbs. Rest of the examination was normal. Brain MRI showed acute infarct in left anteromedial thalamus. MR angiography of intracranial arteries was normal [Figure 4]. Carotid and vertebral arteries Doppler were normal. 2D transthoracic echocardiography showed concentric left ventricular hypertrophy. Serum homocysteine level was normal. She was treated with antiplatelets and statin. At 1-month follow-up, there was a mild improvement in vertical gaze.
- Brain magnetic resonance imaging-diffusion-weighted imaging axial view (a) bright signal change in left anteromedial thalamus (red arrow); apparent diffusion coefficient axial view (b) corresponding dark signal change (red arrow); magnetic resonance angiography of intracranial arteries shows no abnormality (c)
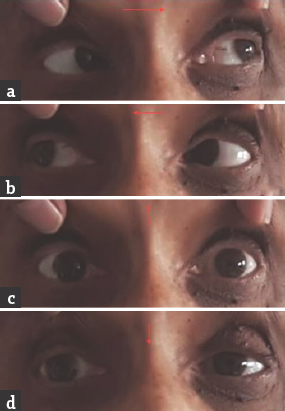
- (a and b) Normal horizontal eye movements; (c and d) restricted vertical eye movements
Case 3: A 67-year-old male presented with a history of giddiness and vomiting of 1-day duration. Giddiness was in the form of spinning sensation, more severe on upright position and associated with vomiting. There was no dysarthria, dysphagia, nasal regurgitation, motor weakness, or sensory abnormalities. He was diabetic on medications with poor glycemic control. General physical and systemic examination was normal. His pulse rate was 92/min and blood pressure was 160/90 mmHg. He was conscious and oriented. Speech was normal. Fundus examination was normal. Pupils were equal and reactive. Right homonymous hemianopia was present. Oculomotor abnormalities were noted in the form of restricted up and down gaze in both eyes. Vertical vestibulo-ocular reflex was preserved. Convergence was restricted on both sides. Horizontal gaze was preserved in both eyes [Figure 5]. Rest of the cranial nerves was normal. Rest of examination was normal. Brain MRI showed acute infarct in left anteromedial thalamus and occipital cortex. MR angiography showed thinning of both PCAs [Figure 6]. Carotid and vertebral arteries' Doppler was normal. 2D transthoracic echocardiography showed concentric left ventricular hypertrophy. Glycated hemoglobin was 9.6%. He was treated with antiplatelets and statin. At 3-month follow-up, his oculomotor abnormalities got normalized.
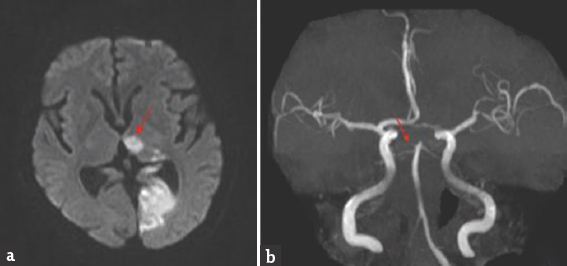
- Brain magnetic resonance imaging-diffusion-weighted imaging axial view (a) bright signal change in the left thalamus (red arrow) and occipital cortex; magnetic resonance angiography (b) shows thinning of both posterior cerebral artery
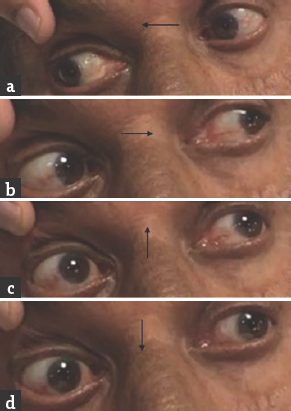
- (a and b) Normal horizontal eye movements; (c and d) restricted vertical eye movements
The coexistence of paramedian thalamic and rostral midbrain infarction is due to their vascular supply; a single paramedian artery arising near the top of the basilar branch to supply both the paramedian thalamus (paramedian thalamic artery) and the rostral medial mesencephalon (paramedian peduncular artery). Alternatively, isolated paramedian thalamic or midbrain infarcts can occur in individuals in whom the paramedian peduncular arteries arise separately from the paramedian thalamic arteries.[7] In the mesencephalic reticular formation lays the neural structures responsible for mediating vertical eye movements. The supranuclear pathways for vertical gaze are not well understood, unlike those for horizontal eye movements. There are various vertical eye movement abnormalities reported due to thalamo-mesencephalic infarction. Iijima et al. reported a patient with up and down gaze palsy that had unilateral thalamo-mesencephalic infarct involving riMLF sparing PC.[8] Bogousslavsky and Meienberg (1984) reported the occurrence of bilateral upgaze palsy and monocular downward gaze paresis in ipsilateral thalamo-mesencephalic infarction and named it as vertical “one-and-a-half” syndrome.[1] Unilateral riMLF lesion can explain bilateral supranuclear upgaze palsy as fibers mediating upgaze traverses through opposite riMLF before innervating bilateral elevator muscles. Similarly, fibers mediating downgaze traverses through opposite riMLF before innervating ipsilateral downgaze muscles. Ipsilateral riMLF lesions can cause ipsilateral or contralateral downgaze palsy. Unilateral riMLF lesion causes vertical “one-and-a-half” syndrome. Deleu et al. reported the occurrence of bilateral conjugate downward palsy and monocular upgaze palsy as vertical “one-and-a-half” syndrome.[9] Patient had bilateral riMLF lesions causing bilateral conjugate downward palsy. Ahdab and Riachi reported a patient with ipsilateral downward gaze palsy and contralateral upgaze palsy due to thalamo-mesencephalic infarction and named it as “vertical half-and-a-half” syndrome.[6] Ipsilateral downward gaze palsy was due to ipsilateral riMLF lesion. It interrupted the ipsilateral projections to the eye depressor muscles. The contralateral upward gaze palsy was probably due to injury to the premotor fibers innervating contralateral elevator muscles due to thalamic lesion.
Our first patient had bilateral supranuclear vertical gaze palsy, and imaging showed acute infarct in unilateral thalamo-mesencephalic junction. Bilateral upgaze palsy can be explained due to unilateral riMLF lesion as described earlier. Unilateral riMLF can cause ipsilateral downward gaze palsy. Contralateral downward gaze palsy may be due to lesion in the thalamus. Bogousslavsky et al. reported a female who was in locked-in state due to infarction of ventral pons. Subsequently, she had complete vertical gaze palsy for voluntary and pursuit movements with selective unilateral riMLF infarction.[10] Alemdar et al. reported a female with sudden onset bilateral vertical gaze palsy. She had unilateral infarct involving riMLF and INC sparing PC.[11] Similarly, Suzuki et al. reported a patient who had vertical one-and-a-half syndrome due to unilateral thalamo-mesencephalic infarct progressed to cause conjugate upgaze and downgaze palsy with the extension of infarct into the dorsal portion of the midbrain.[12]
Our second and third patient had bilateral supranuclear vertical gaze palsy, and imaging showed acute infarct in the unilateral anteromedial thalamus. Thalamic lesions can produce acute vertical gaze palsy. The vertical gaze abnormality in paramedian thalamic infarctions is speculated to be due to interruption of supranuclear fibers from the frontal eye fields traversing through the medial thalamus en route to the prerubral and pretectal areas. Some of the frontocortical fibers may decussate in the medial thalamus.[7] Clark and Albers reported three patients with medial thalamic infarction who had acute vertical gaze palsy.[7] Khan et al. reported a patient with unilateral medial thalamic infarct who had vertical gaze palsy and skew deviation.[13] Unilateral thalamic infarct without the involvement of midbrain causing bilateral supranuclear vertical gaze palsy has not been reported.
Unilateral thalamo-mesencephalic infarct is known to cause vertical gaze abnormality. Similarly, thalamic lesion also causes vertical gaze abnormality. Bilateral complete vertical gaze palsy is rare due to unilateral thalamo-mesencephalic and thalamic infarct. We name this vertical gaze abnormality as “vertical one-and-a-one” syndrome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Eye-movement disorders in brain-stem and cerebellar stroke. Arch Neurol. 1987;44:141-8.
- [Google Scholar]
- A hypothetical scheme for the brainstem control of vertical gaze. Neurology. 2000;54:1985-93.
- [Google Scholar]
- Upgaze palsy and monocular paresis of downward gaze from ipsilateral thalamo-mesencephalic infarction: A vertical “one-and-a-half” syndrome. J Neurol. 1984;231:43-5.
- [Google Scholar]
- Coexisting vertical and horizontal one and a half syndromes. J Neurol Neurosurg Psychiatry. 2000;69:401-2.
- [Google Scholar]
- Neurological picture. Vertical 'half-and-a-half' syndrome. J Neurol Neurosurg Psychiatry. 2012;83:834-5.
- [Google Scholar]
- Vertical gaze palsies from medial thalamic infarctions without midbrain involvement. Stroke. 1995;26:1467-70.
- [Google Scholar]
- A case of vertical gaze palsy associated with a unilateral infarct in the thalamo-mesencephalic junction on MR imaging. Rinsho Shinkeigaku. 1994;34:356-60.
- [Google Scholar]
- Vertical one-and-a-half syndrome. Supranuclear downgaze paralysis with monocular elevation palsy. Arch Neurol. 1989;46:1361-3.
- [Google Scholar]
- Vertical gaze palsy and selective unilateral infarction of the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) J Neurol Neurosurg Psychiatry. 1990;53:67-71.
- [Google Scholar]
- Unilateral midbrain infarction causing upward and downward gaze palsy. J Neuroophthalmol. 2006;26:173-6.
- [Google Scholar]
- Case of unilateral thalamo-mesencephalic infarction with enlargement to bilateral vertical gaze palsy due to vertical one-and-a-half syndrome. Brain Nerve. 2008;60:92-6.
- [Google Scholar]
- Unilateral thalamic infarction presenting as vertical gaze palsy: A case report. J Med Case Rep. 2011;5:535.
- [Google Scholar]





