Translate this page into:
A Case Series of Fetal Valproate Syndrome in the Republic of Crimea
Ching Soong Khoo MRCP (UK), Jalan Yaacob Latif, Bandar Tun Razak 56000 Cheras, Kuala Lumpur, Malaysia chingsoongkhoo@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Valproic acid or valproate is a well-recognized potent teratogen. Antenatal exposure to this drug can cause fetal valproate syndrome (FVS), which is a constellation of distinctive dysmorphic features and congenital malformations. Despite an abundance of reports and registries about this syndrome, there is lack of information from Russia, in particular, the Republic of Crimea. We herein describe two cases of FVS from our registry.
Keywords
fetal valproate syndrome
antiepileptic drug
valproic acid
pregnancy
Introduction
Valproic acid (VPA) is commonly prescribed in patients with epilepsy because of its broad spectrum of antiepileptic efficacy. Its anticonvulsant effects are believed to be related to blockade of voltage-gated sodium channels and elevated brain concentrations of gamma-aminobutyric acid.1 In view of its ability to cross the blood–placental barrier, VPA use during pregnancy may cause fetal valproate syndrome (FVS), which is characterized by distinctive facial dysmorphism, congenital anomalies, and neurodevelopmental delay.
In Europe, FVS was reported to have a significant annual decrease by 27.5%.2 It might be due to increasing awareness of prescribing VPA in childbearing or pregnant women, or underreporting and underrecognizing.
Data from Russia on FVS are scarce. Crimea, as part of Russia, is a place of exceptional ethnic diversity. It is thus worthwhile describing the clinical features of FVS from the Crimean registry.
Case History
Case 1
The first case was a 10-day-old female neonate born to nonconsanguineous parents. Her mother had been taking VPA for epilepsy for almost 11 years and was on a daily dose of 1,200 mg throughout her pregnancy. She took folate at 400 mg concurrently. The infant was born at full term.
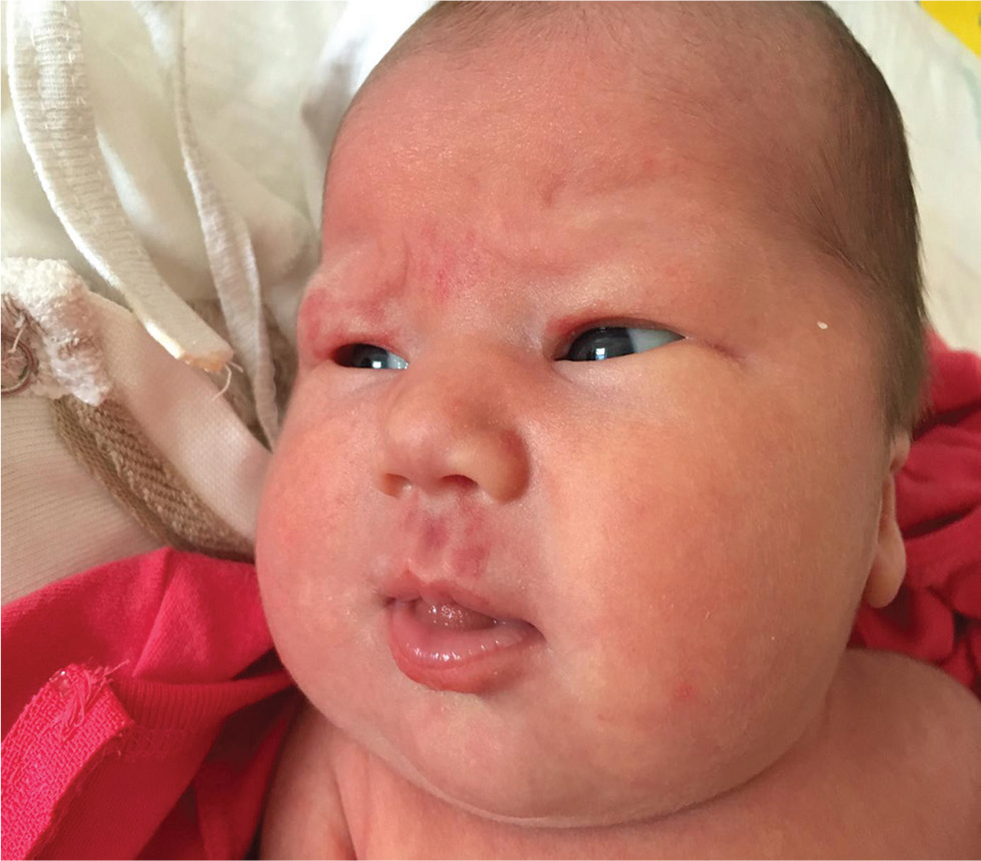
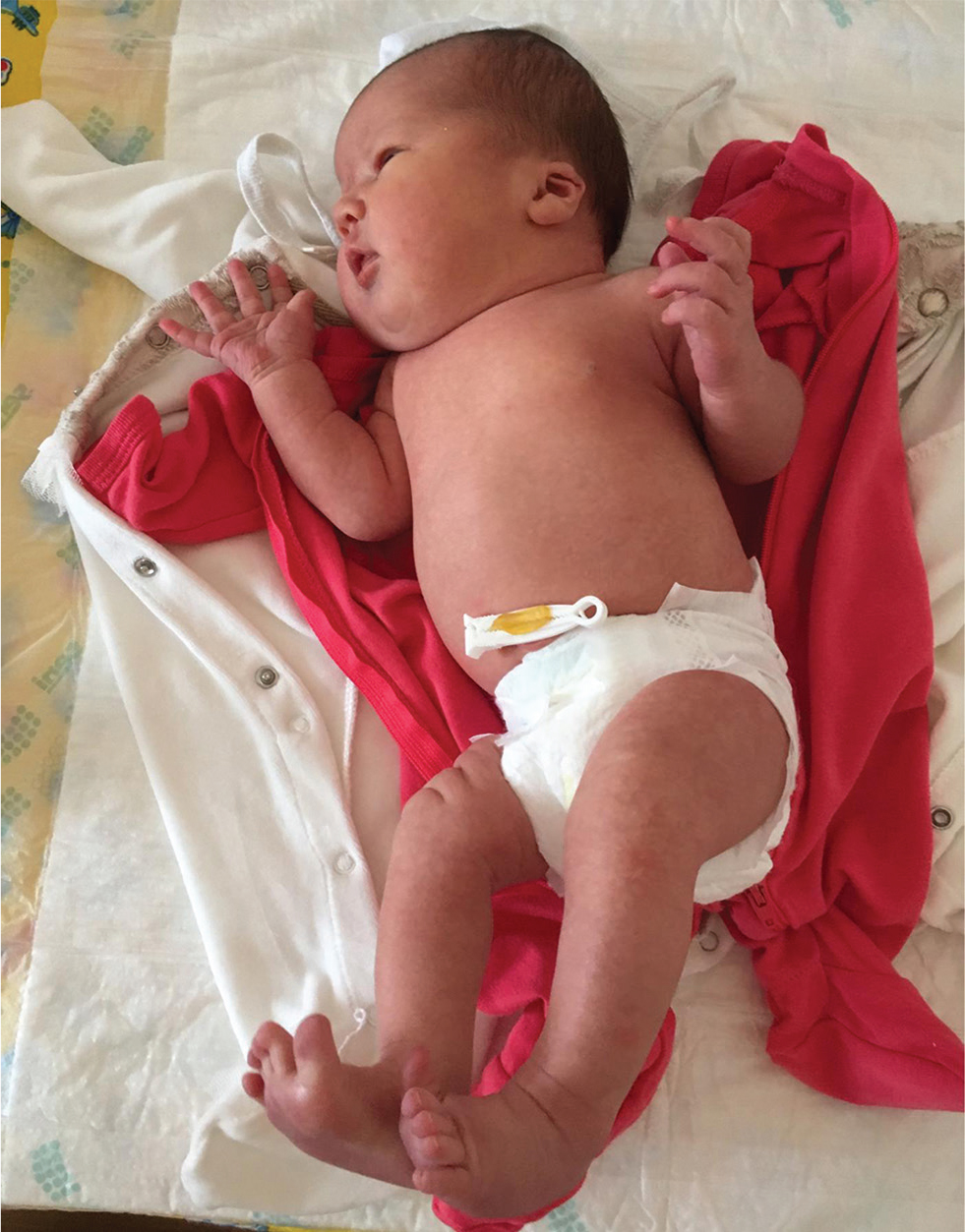
She had dysmorphic facial features with a high and broad forehead; epicanthic folds; thin, arched, and wide-spaced eyebrows; a small, upturned nose with a wide nasal bridge; a long, shallow philtrum; a thick lower lip, low-set ears, small mouth, and eyebrow hair loss; and hemangiomas on the upper lip and right superior eyelid (Fig. 1). She had broad hands and feet; bilateral camptodactyly and clubbed feet (Fig. 2). On examination, her chest was barrel-shaped with a short neck. Mild systolic cardiac murmurs were auscultated.
-
Fig. 1 Dysmorphic features of the first case.
Fig. 1 Dysmorphic features of the first case.
-
Fig. 2 Short neck and barrel-shaped chest wall in the first case.
Fig. 2 Short neck and barrel-shaped chest wall in the first case.
Her brain magnetic resonance imaging (MRI) revealed multilobar hypoplasia, porencephalic cysts, and corpus callosum agenesis. Echocardiogram (ECHO) detected a ventricular septal defect. Abdominal ultrasound was normal. Electroencephalogram revealed epileptic discharges from the right occipital region.
Case 2
A 3-month-old female infant was brought to the tertiary hospital with myotonia, psychomotor delay, and malnutrition. She was born to nonconsanguineous parents. Her mother had been taking VPA for the past 15 years and was on a daily dose of 800 mg throughout her pregnancy. She was compliant with folate at 800 mg daily. Polyhydramnios was detected at 31 weeks of gestation. The infant was born at full term.
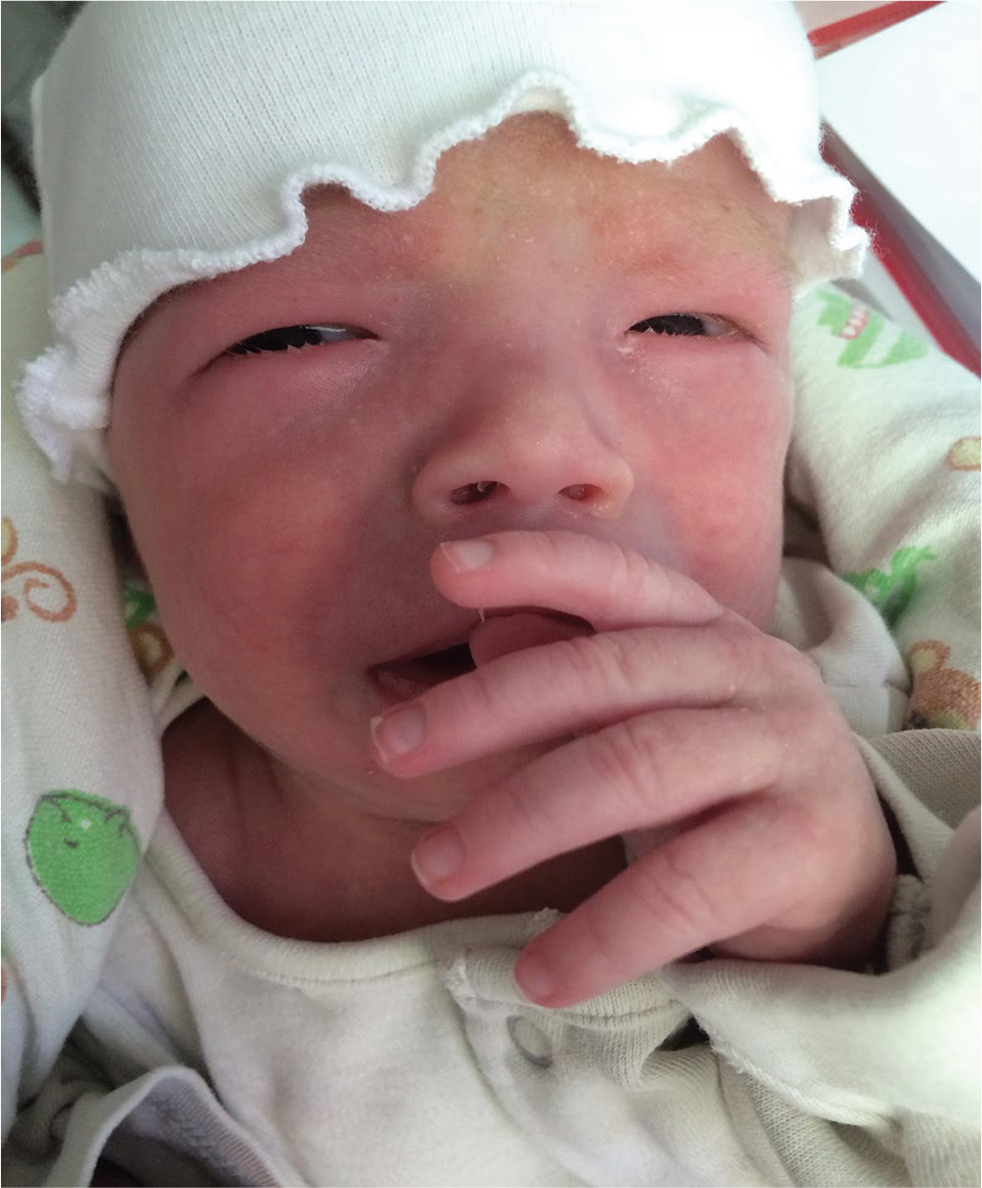
On examination, her weight and length were 5.1 kg and 53 cm, respectively (below the fifth centile for age and sex) in keeping with failure to thrive. She had a high and broad forehead; epicanthic folds; thin, arched, and wide-spaced eyebrows; a small and upturned nose; a long and shallow philtrum; and a thick lower lip, low-set ears, and small mouth (Fig. 3). Bilateral fifth toe hypoplasia, toe angulation deformities, broad hands and feet, bilateral clubbed fingers, and loose skin were seen. She had pectus excavatum. Cardiovascular examination revealed loud systolic cardiac murmurs.
-
Fig. 3 Dysmorphic features of the second case.
Fig. 3 Dysmorphic features of the second case.
MRI of the brain and spinal cord was grossly normal. ECHO showed a persistent left superior vena cava (PLSVC) draining into the coronary sinus with mild pulmonary hypertension.
In both cases, the screening for toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, human immunodeficiency virus, syphilis, and hepatitis B and C infections were negative. Work-up for inherited metabolic and mitochondrial diseases was negative.
Discussion
One of the most common anomalies attributed to VPA exposure is neural tube defects (NTDs), which were reported up to 5%.3 4 However, none of our case series had spine defects. These findings were in agreement with a previous study with no NTDs in their cases.5 Cardiac malformations are commonly reported in FVS,6 which were also observed in both our cases. Interestingly, one of our infants had PLSVC, whose association with FVS was not described in any literature. Musculoskeletal abnormalities have recently been highlighted in relation to VPA exposure.7 From our case series, one had camptodactyly and clubbed feet, and another one had toe angulation deformities and clubbed fingers.
A recent report demonstrated that the risks of major birth anomalies are not only influenced by a certain type of antiepileptic drug but also by its dose.8 Higher malformation rates were seen in those mothers taking 1,500 mg or more per day.8 A daily dose of less than 700 mg seemed to be of a similar risk compared with other antiepileptic drugs; however, the authors could not draw a conclusion that this could be a safer dose. Cutoff values to determine higher risks were reported in several studies ranging from 600 to 1,500 mg daily. Our last case showed that FVS could occur even at a relatively lower dose (800 mg per day). Mutlu-Albayrak et al reported FVS cases at as low as 500 mg a day.7
Genetic susceptibility to VPA exposure is linked to FVS, which could explain why only certain groups of children are affected. This may include the underlying genetic polymorphism influencing the drug metabolism in both the mother and fetus. This hereditary susceptibility theory is strengthened by a report of FVS in three sets of siblings.5 VPA, as a direct inhibitor of histone deacetylase, was found to induce epigenetic modifications.9 Long-term use of VPA, in addition to genetic fetal susceptibility may help propagate the cascade of VPA teratogenicity. This phenomenon was observed in all mothers taking VPA more than 10 years.7 Our first case took VPA for 11 years, and the second one took it for nearly 15 years.
The role of folate supplementation in preventing FVS remains debatable. In both our cases, FVS occurred despite compliance with folate. The UK registry showed that the rates of major congenital defects in mothers taking antiepileptics on folate were as high as in those on antiepileptic drugs without folate.10 However, these findings did not oppose to the common recommendation of folate use in the pregnant women.
In conclusion, FVS can occur at different doses of VPA. We found PLSVC in one of our cases, which has not been described previously in association with FVS. Meticulous ultrasound examination is crucial during antenatal follow-up for screening of congenital anomalies in pregnant mothers on antiepileptic drugs.
Key Messages: Fetal valproate syndrome can occur at any dose of valproate exposure during pregnancy. Meticulous ultrasound examination is crucial during the antenatal follow- up for screening of congenital anomalies in those mothers taking valproate.
Conflict of Interest
None declared.
Funding None.
References
- A sustainable solution for the activities of the European network for surveillance of congenital anomalies: EUROCAT as part of the EU Platform on Rare Diseases Registration. Eur J Med Genet. 2018;61(9):513-517.
- [Google Scholar]
- Antiepileptic Drug Pregnancy Registry. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64(6):961-965.
- [Google Scholar]
- Valproate embryopathy in three sets of siblings: further proof of hereditary susceptibility. Neurology. 2002;59(4):630-633.
- [Google Scholar]
- EUROCAT Antiepileptic Study Working Group. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362(23):2185-2193.
- [Google Scholar]
- EURAP study group. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10(7):609-617.
- [Google Scholar]
- Epigenetic modifications in valproic acid-induced teratogenesis. Toxicol Appl Pharmacol. 2010;248(3):201-209.
- [Google Scholar]
- Folic acid use and major congenital malformations in offspring of women with epilepsy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2009;80(5):506-511.
- [Google Scholar]






