Translate this page into:
Surgical Management and Outcomes of Aneurysms of Posterior Inferior Cerebellar Artery: Location-Based Approaches with Review of Literature
K. V. L. Narasinga Rao, MCh Department of Neurosurgery, National Institute of Mental Health and Neurosciences, Bengaluru - 560 029 Karnataka India neuronarsi@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Posterior inferior cerebellar artery (PICA) is a tortuous, variable, and uncommon site for aneurysms. Surgical management of PICA aneurysms involves careful selection of approach based on the location of the aneurysm and meticulous dissection of the neurovascular structures and perforators.
Materials and Methods We did a retrospective review of all the PICA aneurysms operated at our institute in the past 10 years along with the site, presentation, and approach used for the same. Preoperative World Federation of Neurosurgical Society scores and follow-up modified Rankin scores (mRS) were also evaluated. During the same period, data for intervention cases of PICA aneurysm were also collected with follow-ups for a comparative analysis.
Results A total of 20 patients with 21 PICA aneurysms were reviewed. All the reviewed cases presented with subarachnoid hemorrhage, and the most common location was the lateral medullary segment and vertebral artery (VA)–PICA junction. Midline approaches were used for distal PICA cases, with far-lateral approach reserved for anterior medullary/VA–PICA junction. No lower cranial nerve palsies were recorded at follow-up. Four cases needed cerebrospinal fluid diversion and two developed cerebellar infarcts. All cases were mRS 0 to 2 at follow-up.
Conclusion Our series compares well with some of the larger surgical series of PICA aneurysms. This may be due to early referral patterns and early surgery (<24 hours) policy at our institution. Anatomical knowledge of PICA anatomy and sound perioperative management are keys to good outcomes in these cases.
Keywords
far-lateral approach
posterior inferior cerebellar artery aneurysm
subarachnoid hemorrhage
suboccipital approach
Introduction
Posterior circulation aneurysms arise from vertebral arteries (VAs), basilar arteries (BAs), or their branches. Aneurysms of the posterior inferior cerebellar artery (PICA) comprise 0.5 to 3% of all intracranial aneurysms. They, however, pose a significant risk because of their high morbidity, mortality, and rupture rate. Furthermore, re-bleeding rates are high (78%) primarily due to their relatively thin aneurysm wall and dissecting nature.1 Microsurgical approach is limited by anatomical corridors of brain stem, petrous occipital bone, and multiple neurovascular structures occupying the cerebellomedullary and cerebellopontine cisterns.2 Adding to our misery is the fact that PICA is the most variable artery in terms of its course among all arteries of the posterior circulation.3 The first ligation of cervical VA due to an intracranial aneurysm was reported by Dandy in 1928.4 In 1947, Rizzoli and Hayes trapped and excised a PICA aneurysm.5 In recent years, the trend of treatment of posterior circulation aneurysms has tilted toward the endovascular arm.
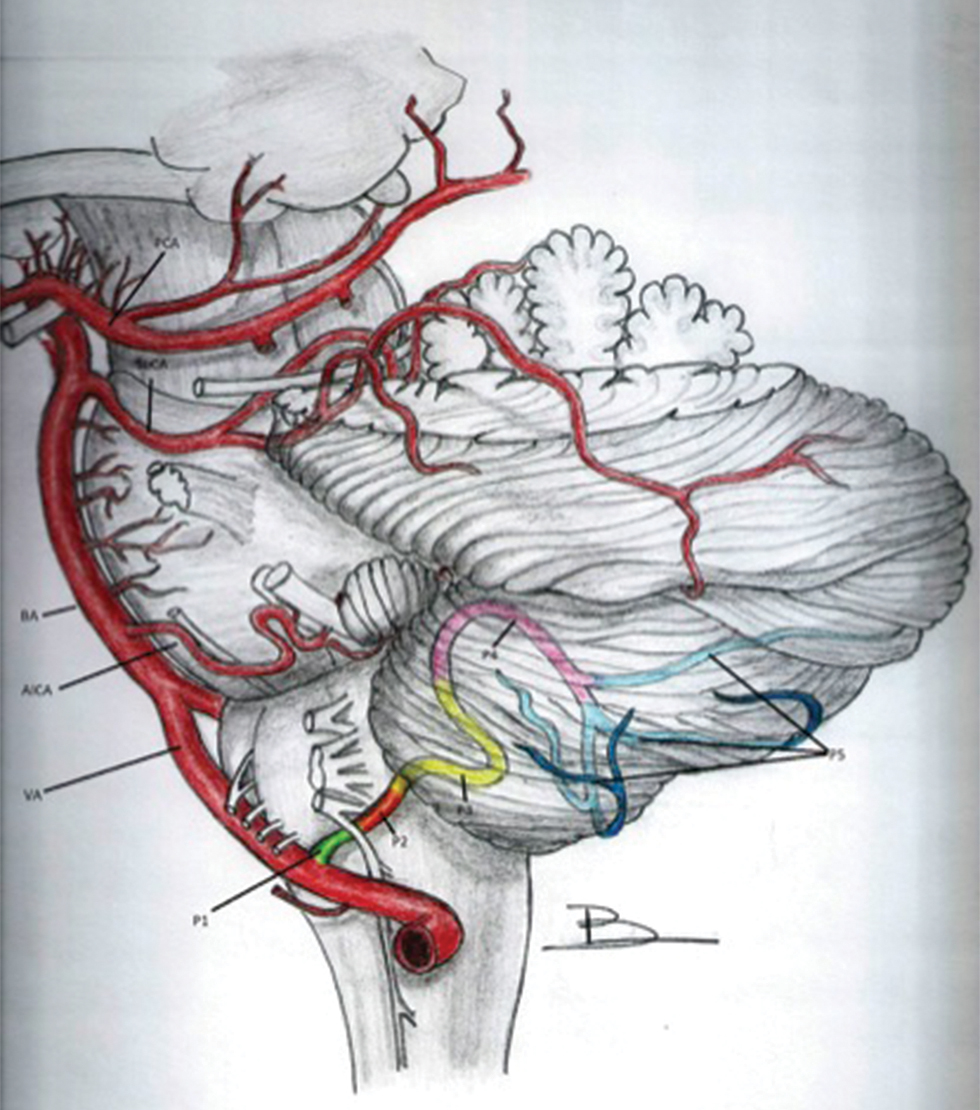
PICA usually originates from the V4 segment, while extracranial origin from V3 has been described. Aneurysms from PICA can originate from one of its six segments and two loops (based on its relationship with the medulla oblongata and the cerebellum) that include (a) the VA-PICA junction, (b) the anterior medullary segment extending from VA-PICA’s origin to the inferior olivary prominence, (c) the lateral medullary segment extending till the origin of IX-X-XI cranial nerves (CNs) from the brain stem, (d) the tonsillomedullary segment extending till the caudal portion of tonsil (including the caudal loop), (e) the telovelotonsillar segment which extends from the midportion of its ascent along the medial surface of tonsil to the cortical cerebellar surface (including the cranial loop), and (f) the cortical segment, extending till the cerebellar vermis and hemisphere (Fig. 1).3 6
-
Fig. 1 Schematic representation of the normal anatomy of (left) posterior inferior cerebellar artery. AICA, anterior inferior cerebellar artery; BA, basilar artery; P1, anterior medullary segment; P2, lateral medullary segment; P3, tonsillo-medullary segment; P4: telovelotonsillar segment; P5, cortical segment; PCA, Posterior cerebellar artery; SuCA, superior cerebellar artery; VA, vertebral artery.
Fig. 1 Schematic representation of the normal anatomy of (left) posterior inferior cerebellar artery. AICA, anterior inferior cerebellar artery; BA, basilar artery; P1, anterior medullary segment; P2, lateral medullary segment; P3, tonsillo-medullary segment; P4: telovelotonsillar segment; P5, cortical segment; PCA, Posterior cerebellar artery; SuCA, superior cerebellar artery; VA, vertebral artery.
Here, we retrospectively analyzed 20 such cases of PICA aneurysm operated at our institute, with special emphasis on the presentation and nuances of approach based on the segment of the aneurysm and outcomes.
Materials and Methods
All operated cases of PICA aneurysm from February 2012 to May 2017 were retrospectively reviewed. All aneurysms were identified using digital subtraction angiography (DSA). Data were collected from patient records under the following heads: demography, presentation, World Federation of Neurosurgical Society (WFNS) grade, and computed tomography (CT) findings. Aneurysm location and anatomy with respect to the management strategy and postoperative neurological complications were analyzed in light of their location on DSA. In cases of multiple aneurysms, the site of rupture was decided based on CT findings, size and irregularity of the aneurysm, or findings in surgery or at an autopsy or both. Patient outcomes were analyzed using modified Rankin scores (mRS) at the time of discharge and at follow-up.
Simultaneously, data for PICA aneurysms undergoing intervention were also sought. This included presentation, segment of aneurysm, associated vascular abnormalities and course, and clinical follow-up data. However, as the series has limited numbers on both sides, a direct comparison in the outcomes was not sought.
Because the data have been sourced from a tertiary care hospital in India, cost was one of the major deciding factors in the treatment decision. Only cases deemed unsuitable by the operating surgeon and those with the available resources for intervention were referred for the same, hence the predominance of surgical intervention in this series.
Operative Approach
We usually approach PICA aneurysms using the midline suboccipital route. Proximal control of the VA was attained early by dissecting the vertebral artery intradurally, just after its dural entry. Foramen magnum rim was removed in all cases, but we did not drill the occipital condyle, as it was not considered essential for adequate exposure. The origin of PICA is usually found near the root exit zone of the hypoglossal nerve. Proximal perforators, particularly to the brain stem, often pose a challenge in adequately delineating the aneurysm. The aneurysmal neck was defined by sharp dissection while being mindful on the critical neurovascular structures surrounding it. The course and tortous nature of the VA-BA complex needs to be considered when deciding the approach to the aneurysm, that is, use either the midline sub occipital craniotomy or the far lateral approach. Intraoperatively, no aneurysmal rupture was recorded. We used the far-lateral approach along with lateral occipital condyle resection in VA-PICA aneurysms (Table 1).
|
Location of aneurysm |
Surgical approach |
Number of aneurysms (n = 21) |
|---|---|---|
|
Abbreviations: BA, basilar artery; PICA, posterior inferior cerebellar artery; VA, vertebral artery. |
||
|
VA/BA–PICA junction/anterior medullary |
Far-lateral approach |
4 |
|
Midline suboccipital craniectomy |
3 |
|
|
Lateral medullary |
Far-lateral approach |
1 |
|
Midline suboccipital craniectomy |
3 |
|
|
Tonsillomedullary/posterior medullary |
Far-lateral approach |
1 |
|
Midline suboccipital craniectomy |
7 |
|
|
Telovelotonsillar |
Far-lateral approach |
None |
|
Midline suboccipital craniectomy |
1 |
|
|
Cortical |
Far-lateral approach |
None |
|
Midline suboccipital craniectomy |
1 |
|
Results
Demographics and Clinical Presentation
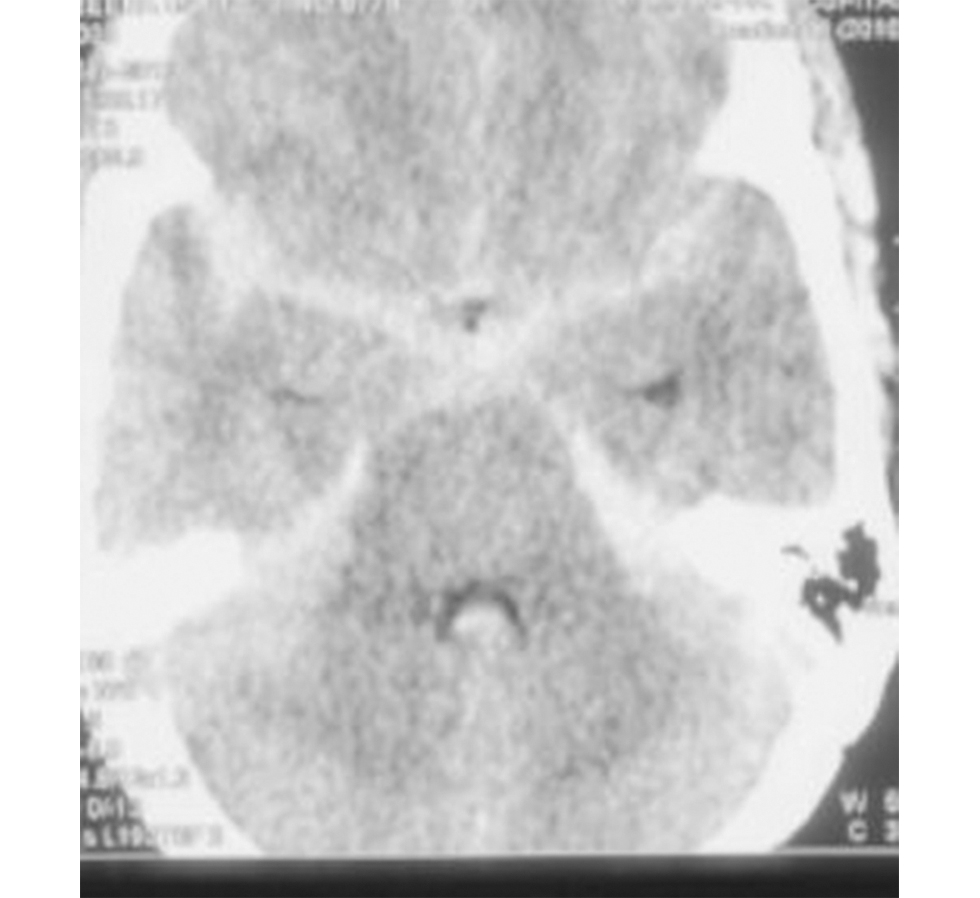
A total of 20 cases with 21 PICA aneurysms were analyzed. In one case with multiple aneurysms, one PICA junctional aneurysm was present along with left superior hypophyseal artery and left communicating segment ICA aneurysm. Another case had two PICA aneurysms: one in the tonsillomedullary segment and another in the cortical segment. Among all the 20 patients, 12 were female and 8 were male (male: female = 2:3). All patients presented with aneurysmal rupture and subarachnoid hemorrhage (SAH) (Fig. 2) with or without intraventricular hemorrhage (IVH). The mean interval between ictus and surgery was 29 days (range: 1–300 days). As expected, sudden-onset severe “thunderclap” holocranial headache was the presenting feature in 18 patients; 7 patients had transient loss of consciousness and 2 had associated seizures (generalized tonic–clonic seizures). Difficulty in swallowing and hoarseness of voice was present in one patient. Sixteen patients had WFNS Grade 1, two patients had Grade 2, and two patients had Grade 4 (Table 2).
-
Fig. 2 Preoperative plain computed tomography scan showing presentation of a case with extensive subarachnoid hemorrhage.
Fig. 2 Preoperative plain computed tomography scan showing presentation of a case with extensive subarachnoid hemorrhage.
|
Age and sex |
Symptoms at presentation |
WFNS grade |
Interval from ictus (days) |
Aneurysmal morphology |
Postoperative status |
|---|---|---|---|---|---|
|
Abbreviation: WFNS, World Federation of Neurosurgical Society. |
|||||
|
53 and female |
Headache and vomiting |
1 |
4 |
Bilobed aneurysm |
No new deficits |
|
38 and female |
Headache, transient loss of consciousness, and difficulty in swallowing |
1 |
23 |
Multilobed aneurysm |
Right lower cranial nerve palsy persistent |
|
53 and female |
Headache and vomiting |
2 |
4 |
Saccular aneurysm |
No new deficits |
|
70 and male |
Headache and vomiting |
1 |
4 |
Saccular aneurysm |
No new deficits |
|
65 and female |
Headache, vomiting, and transient loss of consciousness |
4 |
2 |
Saccular aneurysm |
No new deficits |
|
54 and female |
Headache and vomiting |
2 |
2 |
Saccular aneurysm |
No new deficits |
|
45 and male |
Headache |
1 |
6 |
Saccular aneurysm |
Left hemiparesis |
|
70 and male |
Headache, vomiting, and neck stiffness |
1 |
2 |
Saccular aneurysm |
Bilateral cerebellar symptoms |
|
53 and male |
Headache, vomiting, and seizure |
1 |
15 |
Fusiform |
No new deficits |
|
53 and female |
Headache and transient loss of consciousness |
4 |
3 |
Fusiform |
No new deficits |
|
52 and male |
Headache |
1 |
10 months |
Saccular aneurysm |
No new deficits |
|
45 and female |
Headache and vomiting |
1 |
1 |
Multiple lobes |
No new deficits |
|
35 and female |
Headache |
1 |
6 months |
Multiple lobes |
No new deficits |
|
35 and male |
Transient loss of consciousness |
1 |
1 |
Saccular aneurysm |
No new deficits |
|
52 and male |
Headache and transient loss of consciousness |
1 |
15 |
Saccular aneurysm |
No new deficits |
|
46 and female |
Headache, vomiting, and neck stiffness |
1 |
7 |
Saccular aneurysm |
No new deficits |
|
58 and female |
Headache, vomiting, and transient loss of consciousness |
1 |
5 |
Saccular aneurysm |
No new deficits |
|
65 and female |
Headache and vomiting |
1 |
2 |
Saccular aneurysm |
No new deficits |
|
55 and male |
Giddiness |
1 |
2 |
Saccular aneurysm |
No new deficits |
|
77 and female |
Headache and vomiting |
1 |
2 |
Saccular aneurysm |
No new deficits |
Radiological Analysis
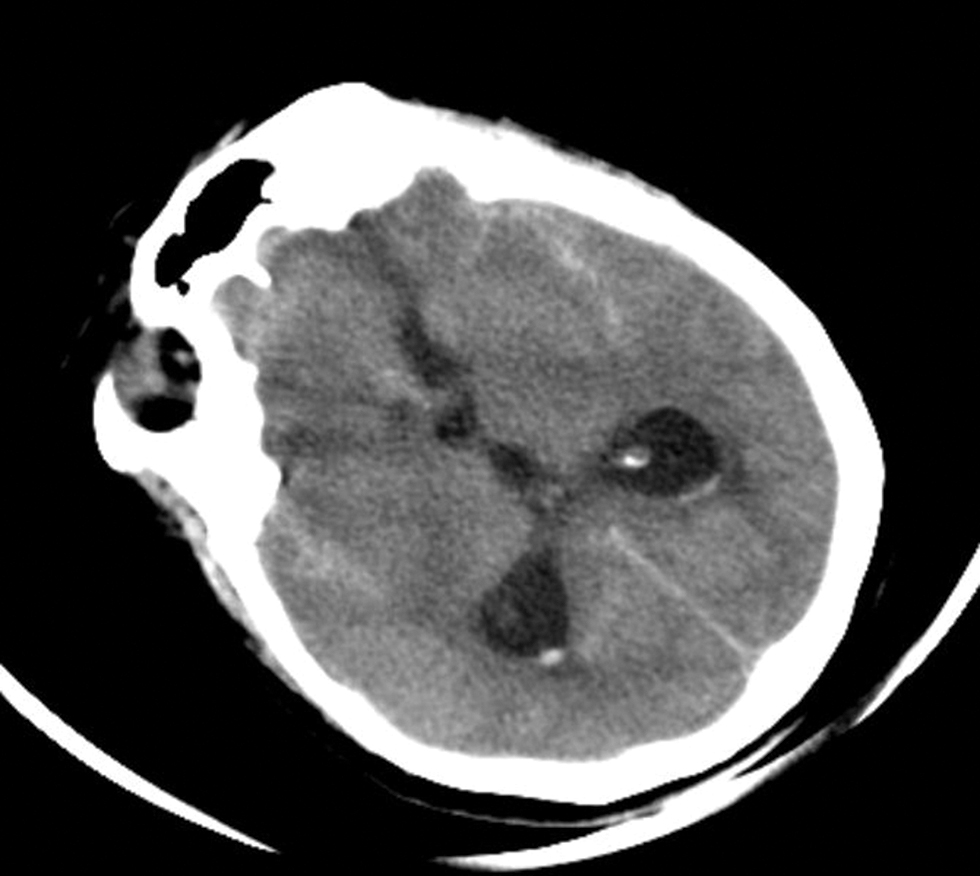
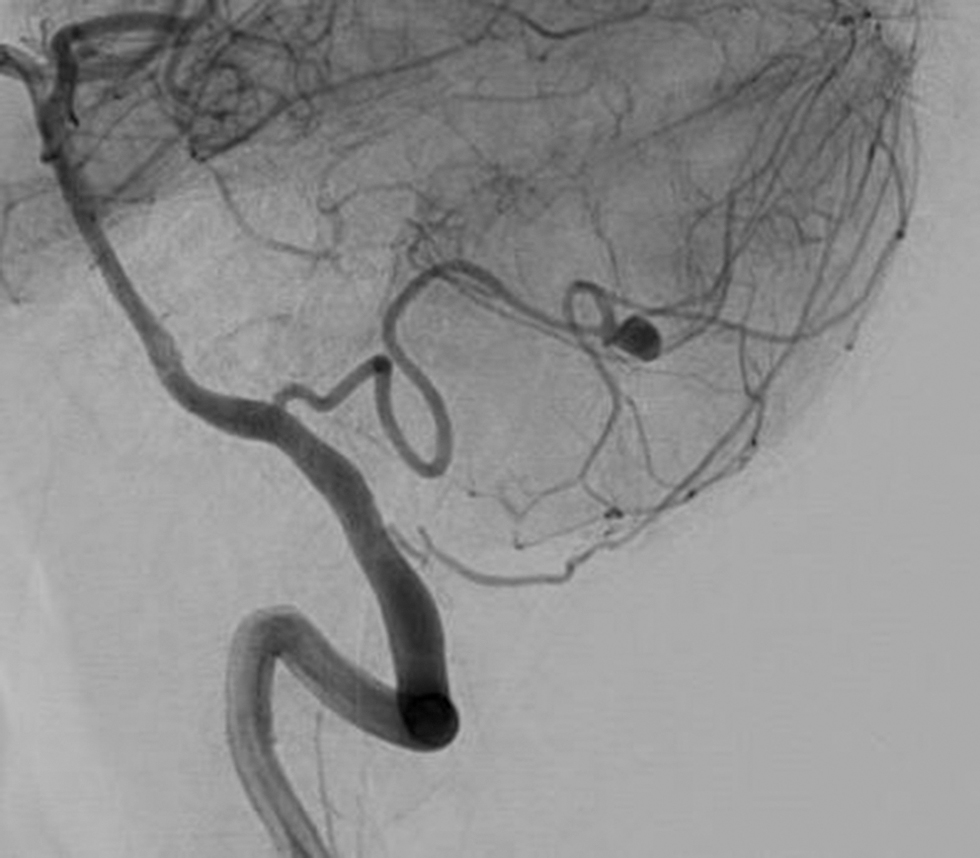
All the patients who presented with acute symptoms suggestive of aneurysmal rupture underwent CT within 24 to 48 hours after the ictus (Table 3). Hydrocephalus was present in six (30%) patients (Fig. 3). Of all the cases only one required preoperative external ventricular drain and another patient needed cerebrospinal fluid (CSF) drainage intraoperatively which was carried over postoperatively as well (Fig. 4A). We had one case of junction aneurysm with retrograde filling in left VA. In one case with multiple aneurysms, one PICA junction aneurysm was present along with left superior hypophyseal artery and left communicating segment ICA aneurysm (Fig. 4B). One case of double aneurysm was also present with a tonsillomedullary segment aneurysm and another in the cortical segment (Fig. 5). Only one aneurysm was giant (>25 mm) in size.
|
Feature on plain CT |
Number of patients (n = 20), n (%) |
|---|---|
|
Abbreviations: CT, computed tomography; HCP, hydrocephalus; IVH, intraventricular hemorrhage; SAH, subarachnoid hemorrhage. |
|
|
SAH |
8 (40) |
|
Bleed in supra- and infratentorial cistern |
4 (20) |
|
IVH |
16 (80) |
|
Exclusively fourth ventricular blood |
5 (25) |
|
Lateral, third and fourth ventricular bleed |
11 (55) |
|
HCP |
6 (30) |
|
Cerebellar bleed |
3 (15) |
-
Fig. 3 Preoperative plain computed tomography scan showing presentation of case with subarachnoid hemorrhage and preoperative hydrocephalus mandating extraventricular drain placement.
Fig. 3 Preoperative plain computed tomography scan showing presentation of case with subarachnoid hemorrhage and preoperative hydrocephalus mandating extraventricular drain placement.
-
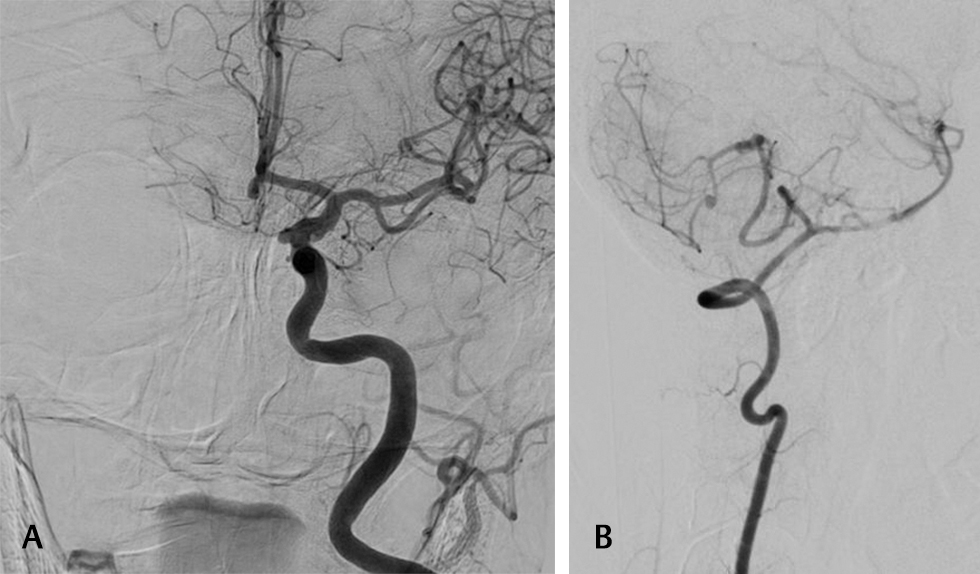
Fig. 4 Preoperative digital subtraction angiography showing saccular aneurysm in the 4th segment of posterior inferior cerebellar artery.
Fig. 4 Preoperative digital subtraction angiography showing saccular aneurysm in the 4th segment of posterior inferior cerebellar artery.
-
Fig. 5 (A) Preoperative digital subtraction angiography of multiple aneurysm case showing medially directed supraclinoid inferior cerebellar artery aneurysm and another anterior communicating segment aneurysm. (B) Preoperative digital subtraction angiography of multiple aneurysm case showing a double posterior inferior cerebellar artery aneurysm, with one in the tonsillomedullary segment and the other in the cortical segment.
Fig. 5 (A) Preoperative digital subtraction angiography of multiple aneurysm case showing medially directed supraclinoid inferior cerebellar artery aneurysm and another anterior communicating segment aneurysm. (B) Preoperative digital subtraction angiography of multiple aneurysm case showing a double posterior inferior cerebellar artery aneurysm, with one in the tonsillomedullary segment and the other in the cortical segment.
Presurgical Management
In patients admitted within 3 weeks of ictus, oral nimodipine along with mannitol was started. Following angiography to confirm the location and morphology of the aneurysm, surgery was performed as early as possible. The surgical approaches included midline suboccipital craniotomy (n = 15) for aneurysms located near the midline and far lateral approach (n = 5) for aneurysms located anterior to the brainstem and at the BA VA PICA junction.
Complications and Outcome
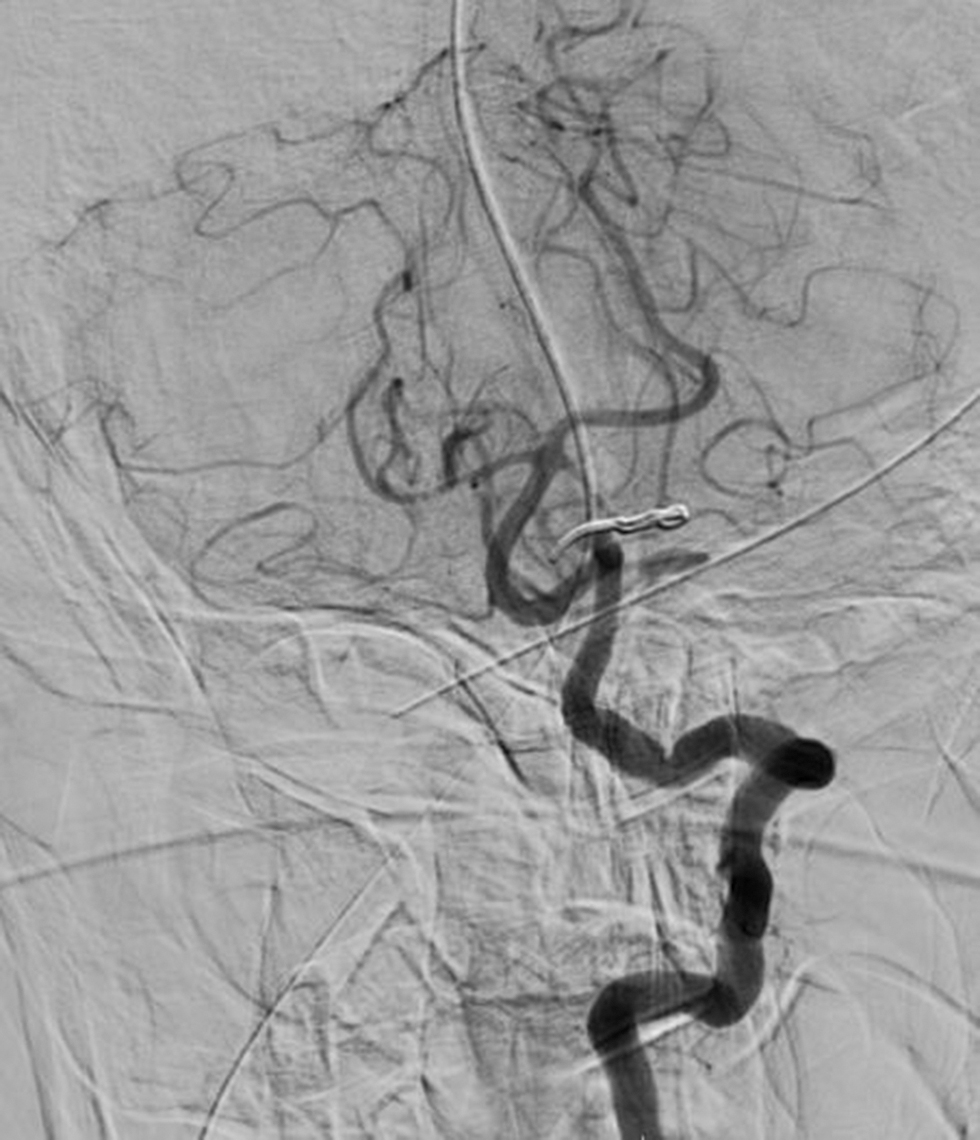
All the 20 patients were available for follow-up. Nearly 75% (15/20) of the patients were functionally independent (mRS = 0–2) at the time of discharge. Among the remaining five cases, three cases improved to mRS of 0 to 2. The mean duration of hospital stay was 8.15 days (range: 5–21 days). Eighteen patients were discharged home and two required rehabilitation. Around 20% (4) of the patients had new postoperative neurological deficits comprising neurological complications. Nearly 10% (2) of the patients developed left-sided weakness that improved with physiotherapy to >3/5. The other 10% (2) of patients developed new postoperative cerebellar signs. One patient who had presented with lower CN symptoms continued to remain the same postoperatively with mild improvement subjectively at 6-month follow-up visit. Both cases were operated via a midline suboccipital craniotomy approach, and one developed left cerebellar infract detected on CT. Four cases (20%) required ventriculo peritoneal shunting which includes 2 cases with EVD placement (see above) and 2 others who presented after 1 month with headache and vomiting with CT showing ventricular dilatation. DSA/CT angiography was performed in all cases either immediately on postoperative day 1 or on follow-up at 6 weeks, depending on the surgeons preference (Fig. 6A). None of the angiograms revealed any evidence of residual aneurysm.
-
Fig. 6 Postoperative digital subtraction angiography showing clip in situ with complete obliteration of aneurysm sac.
Fig. 6 Postoperative digital subtraction angiography showing clip in situ with complete obliteration of aneurysm sac.
Comparison to Intervention
During the same period, a total of five PICA aneurysms underwent coiling. All cases presented with SAH and were investigated with angiography. As the experience, of managing PICA aneurysms at our institute has grown the treatment of more proximally placed PICA aneurysms has shifted toward coiling, a trend which can be made out with the cases treated by endovascular methods (Table 4).
|
Serial number |
Age/sex |
Presentation |
Location of aneurysm |
Course at hospital |
Deficits at last follow-up |
|---|---|---|---|---|---|
|
Abbreviations: AVM, arteriovenous malformation; HCP, hydrocephalus; PICA, posterior inferior cerebellar artery; VA, vertebral artery. |
|||||
|
1 |
40/female |
IV ventricle hemorrhage with HCP |
VA-PICA origin |
Postcoiling persistent HCP, underwent shunt |
12 months, no deficits |
|
2 |
60/female |
III and IV ventricle blood |
VA-PICA origin |
Uneventful |
12 months, no deficits |
|
3 |
55/female |
Ambient cistern and IV ventricle blood |
Lateral medullary segment |
Uneventful |
3 months, no deficits |
|
4 |
58/female |
Blood in lateral and IV ventricles |
Anterior medullary segment |
Uneventful |
24 months, no deficits |
|
5 |
57/male |
IV ventricle blood |
Vermian AVM with cortical segment aneurysm |
Developed brain stem infract and with HCP, underwent shunting procedure |
Did not follow up |
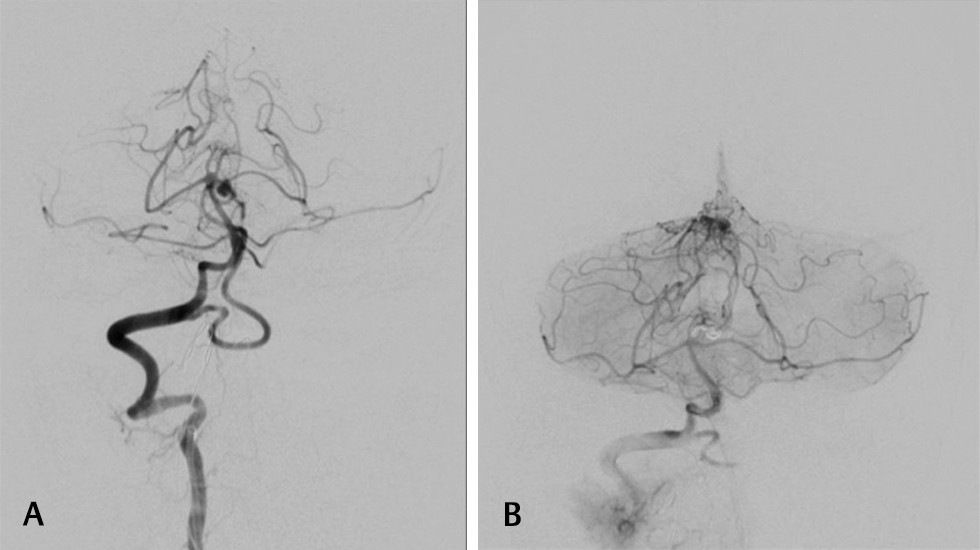
This excludes a single case of cortical segment aneurysm with vermian arteriovenous malformation (AVM) (Figs. 6B 7), where despite being explained the benefit of surgery, the patient opted for intervention. He deteriorated post procedure to E1M3VT status, and subsequent magnetic resonance imaging showed development of early brain stem infarcts and subsequently hydrocephalus. Following shunt surgery, the patient was discharged and did not report to follow-up.
-
Fig. 7 (A) Diagnostic cerebral angiogram of cortical segment posterior inferior cerebellar artery aneurysm with vermian arteriovenous malformation-saccular aneurysm from the 5th segment. (B) Diagnostic cerebral angiogram of cortical segment posterior inferior cerebellar artery aneurysm with vermian arteriovenous malformation.
Fig. 7 (A) Diagnostic cerebral angiogram of cortical segment posterior inferior cerebellar artery aneurysm with vermian arteriovenous malformation-saccular aneurysm from the 5th segment. (B) Diagnostic cerebral angiogram of cortical segment posterior inferior cerebellar artery aneurysm with vermian arteriovenous malformation.
Discussion
The largest review of PICA aneurysms has been conducted by Peerless and Drake7 who reviewed 146 cases of PICA aneurysms and classified outcomes as excellent, good, poor, and dead, without any objectivity in their outcomes. Overall, however, PICA aneurysms are rare (0.49–3.0%) of all intracranial aneurysms. However, these aneurysms present with poor initial neurological status. This is because of the location of this aneurysm. Over 83% of these cases present with IVH as the blood enters from the foramen of Luschka or Magendie and tracks intraventricularly.8 Furthermore, these aneurysms have a high re-bleeding rate (78%) and dissecting nature. In our series too, IVH rates were 80%, while no re-bleeding was encountered. This may be due to our policy to operate within 24 hours of patients’ arrival and early referral of patients to our center.
Demography
In general, PICA aneurysms tend to present at an earlier age as compared with other aneurysms. The mean age as reported in many large series is in the fifth decade, with Lewis et al6 reporting 51 years, Lehto et al9 as 52 years, and Dernbach et al10 even lower at 44.7 years. In their series, Lehto et al9 compared the age of patients with distal PICA aneurysms in eighty cases with those cases who had aneurysms located at other locations during the same study period. They, however, never found a significant difference in the ages, as the mean age of other cases presenting with aneurysms was 49 years. In our series, the mean age was 51 years, ratifying with most previous series.
Aneurysmal Characteristics
PICA has been defined in most series as the most proximal artery originating from the VA and has been divided into five segments, with three medullary segments further branching to variable numbers of perforators of different lengths and trajectories. Four-vessel angiography is the main modality of detection of aneurysm. It is also central to the detection of site, size, other vascular abnormalities, and associated aneurysms. Vascular abnormalities include PICA reduplication, anterior inferior cerebellar artery-supplying PICA territory, absence of opposite artery, and fetal-type circulation. This information is crucial for planning surgical management. Initial angiographic studies can miss the aneurysm, even when the clinical and radiological suspicion is high. Repeat angiography is needed sometimes to clearly understand the anatomy and location of the aneurysm, especially with respect to the lower CNs. One of the aneurysms in our study was missed in initial angiography, which was showed in repeat angiography due to vasospasm.
Most commonly, distal PICA aneurysms arise from lateral medullary segment followed by tonsillomedullary segment as seen in a large series of Tokimura et al11 and Lehto et al9 In our series, however, aneurysms in the tonsillo-medullary segment were the most common site, with 40% (8/20) of all the PICA aneurysms with the anterior medullary or VA PICA junction being the origin of 35% (7/20) aneurysms.
On the basis of morphology, PICA aneurysms have been classified as saccular or fusiform. Dissecting aneurysms have also been described, but their treatment is similar to fusiform aneurysms and hence has been considered together here. Fusiform aneurysms vary from 6 to 62% in various studies,6 9 11 and in our series, it was 10% (2/20).
CT scan of the head shows the site of the bleed, which gives a clue to the location of the aneurysm. Rupture of proximal PICA aneurysms is often diagnosed by the presence of hyperdensity in the ipsilateral basal cisterns that may or may not accompany an extension into the fourth ventricle. Eight (40%) aneurysms of the proximal PICA segments demonstrated SAH with extension into the ventricular system. Patients can have only fourth ventricular hemorrhage with no evidence of any cisternal, cortical, or sulcal blood. This presentation is due to trickling of blood through the foramen of Luschka or Magendie after aneurysmal rupture. These patients almost always have distally located PICA aneurysms. Aneurysms that arise from the tonsillomedullary segment are known to rupture solely into the fourth ventricle.12 In our study, only fourth ventricle hemorrhage was seen in 25% of patients (5/20). Out of these, four cases had distal PICA aneurysm. The incidence of IVH due to PICA aneurysm rupture can range from 83 to 100%,13 14 which also contributes to poorer outcomes due to vasospasm and later development of hydrocephalus. Our series also reports IVH rates of 80% (16/20).
Treatment
Microsurgery remains the bedrock for treating PICA aneurysms, with planning depending on size, location, shape, presentation, and clinical condition of the patient. Midline approaches are safe, familiar, and more reasonable, especially for distal PICA aneurysms. Neurovascular conflicts, proximal perforators, and eloquent areas influence dissection and clipping techniques. Options include direct clipping, clipping with wrapping, wrapping, resection, proximal occlusion or trapping with revascularization, and distal occlusion.9 Dissection and clipping of these aneurysms may lead to lower CN handling and subsequently cause lower CN palsy that even if transient can be very alarming to the patient, especially if not explained prior to the surgery. The rate of lower CN paresis after surgical clipping of proximal PICA aneurysms can vary between 10 and 45%.1 7 In our series, there was only one such case that had presented preoperatively with lower CN symptoms, notably, improving at his 6-month follow-up visit. This is probably because of the early presentation of cases and emergent treatment within a 24-hour window period after presentation at our institute. Furthermore, usually, the related morbidity is self-limiting and will usually improve in ~3 to 6 months.
Although for anterior medullary segment, far-lateral approach provides the best exposure to the cerebellomedullary fissure, the recent trend has been on a tailored approach. Seoane et al15 reported a surgical series using the far-lateral approach to PICA aneurysms without condylar resection. Rodríguez-Hernández and Lawton described the vagoaccessory triangle that is bordered medially by the medulla, laterally by the spinal accessory nerve (CN XI), and superiorly by the vagus nerve (CN X), as an anatomical corridor to reach the PICA aneurysms.2 As for distal PICA aneurysms, midline approach is, without doubt, sufficient. In our series, we have used the far-lateral approach with condylar resection only in 25% (5/20) of the cases, with the majority (⅘) being anterior medullary or VA–PICA junction cases with acceptable complication rates. Other improvised techniques have been described such as the lateral suboccipital approach, the lateral suboccipital transcondylar approach, removal of the posterior condyle, C1 lateral mass, and/or the jugular tubercle, all of which help in widening the surgical corridor.16 Proximal control of VA can be secured by the far-lateral approach and dissection of the arachnoid caudal to the IX and X CNs, avoiding the resection of the posterior lip of the foramen magnum.
Complication in our series was more related to the development of hydrocephalus rather than lower CN paralysis, with 20% (4/20) cases requiring permanent shunting of cerebrospinal fluid (CSF). Recent studies estimate that hydrocephalus, communicating or noncommunicating, is reported in up to 67% of patients with IVH.17 The most plausible theory is that a blood clot blocks the CSF drainage pathway. The phenomenon usually takes place in the narrowest parts of the CSF pathway, namely the cerebral aqueducts or in the outlets of the fourth ventricle. These small and multiple clots can form all over the ventricular lining, causing obstruction to the pathway of CSF via the arachnoid villi leading into the venous sinuses and small blood vessels.18
There have been a few large surgical series describing either distal PICA aneurysms alone or in combination with VA aneurysms (Table 5).
|
Series |
Years |
Number of aneurysms |
Remarks |
Complications (%) |
Major approach (%) |
|---|---|---|---|---|---|
|
Abbreviations: AVM, arteriovenous malformation; BA, basilar artery; CSF, cerebrospinal fluid; HCP, hydrocephalus; PICA, posterior inferior cerebellar artery; VA, vertebral artery. |
|||||
|
Lehto et al9 |
2014 |
91 |
Distal PICA |
18 (22.5) patients died, 52 (65) independent or previous state of living |
Midline suboccipital |
|
Horiuchi et al19 |
2007 |
24 |
Distal PICA |
22 (81.5) clipping 2 (7.4) wrapping 1 (3.7) ligate proximally 1 (3.7) coiling |
Lateral suboccipital approach |
|
Al-Khayat et al20 |
2005 |
52 |
Lower cranial nerve deficits in PICA |
Lower cranial nerve palsy 25 (48.1) |
Lateral suboccipital approach |
|
D’ Ambrosio et al21 |
2004 |
20 |
PICA aneurysms (unruptured) |
Two patients underwent transient vocal cord palsy 3 CSF leak 14 (70) HCP |
Far-lateral approach |
|
Nussbaum et al22 |
2003 |
7 |
Fusiform distal PICA |
Not specified |
Far-lateral approach |
|
Lewis et al6 |
2002 |
20 |
Distal PICA with 6 (30%) AVM |
Shunt 9 (47) Ataxia 5 (26) Dysphagia 2 (10.5) |
Far-lateral transcondylar approach in 19 (86) |
|
Matsushima et al23 |
2001 |
8 |
PICA aneurysms |
||
|
Horowitz et al24 |
1998 |
38 |
PICA aneurysms |
25 (60) new neurological deficit 18 (47) vocal cord palsy 14 (37) dysphagia 1 (3) hearing loss, facial weakness |
Lateral suboccipital approach (84) RMSOC 6 (16) |
|
Bertalanffy et al25 |
1998 |
27 |
PICA aneurysms |
PICA aneurysms |
PICA aneurysms |
|
Sano et al26 |
1997 |
16 |
Dissecting VA aneurysms |
4 good, 1 fair, 1 died 2 excellent, 1 good, 1 fair |
Surgical clipping—6, trapping—5 |
|
Andoh et al27 |
1992 |
38 |
26 junction PICA VA 10 VA 2 VA–BA junction |
Good result—22 (81) |
Not specified |
|
Yamaura et al28 |
1990 |
24 |
Dissecting PICA aneurysms |
Lower cranial nerve—5 (26) Lateral medullary—3 (16) |
19 (79) surgery 10 (52) clipping 7 (36) wrapping |
|
Yamaura29 |
1988 |
86 |
VA aneurysm |
Lower cranial nerve—11 (16) Lateral medullary syndrome—3 (4) Severe deficit—3 (4) |
68 (79) surgical intervention lateral suboccipital approach |
|
Gács et al30 |
1983 |
16 |
PICA aneurysms Six patients associated with AVM |
Good—11 (69) Residual deficit—4 (25) Poor outcome—1 (6) Died—1 (6) |
Not specified |
Endovascular versus Clipping
Recently, the trend has been for endovascular management of PICA aneurysms, due to the concern regarding lower CN morbidity and avoiding critical perforators, especially at the proximal segments. Coil embolization has been the primary method, being most suitable for saccular aneurysms with narrow necks.31 PICA aneurysms have also been treated with flow diverters, although recurrences have been seen because PICA is an end vessel and thus usually without significant collaterals may keep the proximal aspect of the aneurysm patent.32 Endovascular parent vessel occlusion has also been used with success, especially in fusiform and distal aneurysm cases where sacrifice of the parent artery rarely leads to deficits. However, complication rates of 13% have been reported with embolization of PICA.33 This may be due to a multitude of reasons. Superselective catheterization of this tortuous and often variable artery is difficult and may even be impossible for distal aneurysm cases. In addition, with small aneurysms, there is a chance of “jumping” of the catheter or guidewire into the sac, leading to inadvertent rupture. This also leads to an unfavorable anatomy, making it difficult for further coils to be introduced. There may, however, be a place for coiling for the more proximal segments (anterior medullary/VA–PICA junction) as surgical clipping may be tenuous in a bid to preserve brain stem perforators. Another major finding corroborating with previous series is that surgical resection of AVM with aneurysmal clipping is superior to embolization or coiling as can be seen with one case of AVM with aneurysm in our series.31
A situation where neither coiling nor clipping is safe is blister aneurysms of PICA. Here, the outer wall of the aneurysm comprises only the adventitia and/or thrombus that separates the artery from the overlying pia. Here, clipping can cause rupture at the neck of the aneurysm and stent assisted or flow diverter placement is difficult due to the small diameter of the PICA. Parent artery occlusion is often tried but may lead to deficits. Here, a novel technique of endovascular embolization with detachable coils while preserving the parent artery using a combination of soft three-dimensional coil technology and low-profile microcatheter allows good coil positioning, while the microcatheter itself maintains the patency of the parent vessel.34
Another option for these difficult cases is intracranial-to-intracranial (IC–IC) bypass with trapping of the aneurysm. In a largest such series spanning 17 years, Abla et al35 described 129 PICA aneurysms in 125 patients treated microsurgically. A total of 35 IC–IC bypasses were performed as part of PICA aneurysm management. All aneurysms were completely occluded with 94% of bypasses patent. Ischemic complications were seen only in two patients in whom the bypasses occluded, and permanent lower CN morbidity was limited to three patients. They concluded that the PICA aneurysms allow the application of IC–IC bypass better than any other cerebral aneurysms. An algorithmic approach is advocated: trapped aneurysms of the PICA origin (p1 segment) are to be revascularized with a PICA–PICA bypass, with PICA reimplantation being the alternative; trapped p2 segment aneurysms can be reanastomosed, bypassed in situ, or reimplanted; distal p3 segment aneurysms can be reanastomosed or revascularized with a PICA–PICA bypass; and lastly the aneurysms of the p4 segment that are too distal for any of the PICA–PICA bypass can be reanastomosed. Interpositional grafts are to be reserved for when these three primary options are not possible. Such an option is often feasible and provides exceptional results.
Surgical clipping provides what coiling leaves to be desired. Undervision dissection of the aneurysm and avoidance of neurovascular structures are the best method to avoid complications.36 Bohnstedt et al37 published the largest series so far, valuing both microsurgical and endovascular treatments of 102 PICA aneurysms and have favored microsurgical clipping over coiling. Sejkorová et al38 also reported their series of 81 cases of PICA aneurysms during a 15-year period and found more recurrences in the coiled group. A meta-analysis by Petr et al39 of 796 PICA aneurysmal cases comparing clipping and coiling has shown a significantly greater rate of recurrence with coiling (8.1 vs. 1.1%) with no overall long-term differences in clinical outcome between the two treatment strategies. With the experience and review of previous surgical series and our own cases we can safely conclude that if treated early and with sufficient precision morbidity in PICA aneurysms is more likely due to complications related to SAH rather than technical hurdles in clipping the aneurysm..
Limitations
This series suffers from some significant limitations due to it inherently being a retrospective review. The absence of lower CN deficits is very hard to explain, but due to the small numbers and a retrospective review nature, transient lower CN palsies may have been missed. In addition, a far-lateral approach was used in six aneurysms despite 11 out of the 21 aneurysms being located in either the anterior medullary or lateral medullary segments. However, this might be indicative of the current need of less invasive approaches.2 15 16 However, an honest representation of available findings has been attempted along with a thorough review of available literature.
Conclusion
This series, albeit small, provides a bird’s eye view into the microsurgical management of PICA aneurysms with faster referral patterns and early treatment. Lateral medullary segments and VA–PICA junction constitute the most common sites of aneurysms, with midline and lateral approaches being ideal for them, respectively. Dissection if done meticulously prevents lower CN morbidity, which is often transient, if present. Complications are rather due to sequelae of hemorrhage causing hydrocephalus and need for shunting, and thus close follow-up is necessary. Finally, an expert ensemble team of cerebrovascular surgeons and techniques is indispensable to treat these tricky aneurysms.
Conflict of Interest
None declared.
Funding None.
References
- Aneurysms of the medullary segments of the posterior-inferior cerebellar artery: considerations on treatment strategy and clinical outcome. Neurol Sci. 2013;34(4):529-536.
- [Google Scholar]
- Anatomical triangles defining surgical routes to posterior inferior cerebellar artery aneurysms. J Neurosurg. 2011;114(4):1088-1094.
- [Google Scholar]
- Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10(2):170-199.
- [Google Scholar]
- Congenital berry aneurysm of the posterior fossa; case report with successful operative excision. J Neurosurg. 1953;10(5):550-551.
- [Google Scholar]
- Distal posterior inferior cerebellar artery aneurysms: clinical features and management. J Neurosurg. 2002;97(4):756-766.
- [Google Scholar]
- Management of aneurysms of the posterior circulation.Neurological Surgery. Vol Vol. 3. (4th edition.). Philadelphia: W.B. Saunders Co; 1990. p. :806-1764. In: ed.
- [Google Scholar]
- The endovascular management of saccular posterior inferior cerebellar artery aneurysms. Korean J Radiol. 2008;9(5):396-400.
- [Google Scholar]
- Distal posterior inferior cerebellar artery aneurysms: clinical features and outcome of 80 patients. World Neurosurg. 2014;82(5):702-713.
- [Google Scholar]
- Giant and multiple aneurysms of the distal posterior inferior cerebellar artery. Neurosurgery. 1988;22(2):309-312.
- [Google Scholar]
- Clinical presentation and treatment of distal posterior inferior cerebellar artery aneurysms. Neurosurg Rev. 2011;34(1):57-67.
- [Google Scholar]
- Aneurysms of the posterior inferior cerebellar artery. A clinical and anatomical analysis. J Neurosurg. 1983;58(3):381-387.
- [Google Scholar]
- Patterns of hemorrhage with ruptured posterior inferior cerebellar artery aneurysms: CT findings in 44 cases. AJR Am J Roentgenol. 1997;169(4):1169-1171.
- [Google Scholar]
- Bleeding patterns in ruptured posterior fossa aneurysms: a CT study. J Comput Assist Tomogr. 1991;15(4):612-617.
- [Google Scholar]
- Far-lateral approach without drilling the occipital condyle for vertebral artery-posterior inferior cerebellar artery aneurysms. Neurosurgery. 2017;81(2):268-274.
- [Google Scholar]
- Posterior inferior cerebellar artery aneurysms: operative strategies based on a surgical series of 27 patients. Turk Neurosurg. 2014;24(1):30-37.
- [Google Scholar]
- Mechanisms of hydrocephalus after intraventricular haemorrhage in adults. Stroke Vasc Neurol. 2016;1(1):23-27.
- [Google Scholar]
- Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin Neonatol. 2001;6(2):135-146.
- [Google Scholar]
- Characteristics of distal posteroinferior cerebellar artery aneurysms. Neurosurgery. 2003;53(3):589-595, discussion 595–596.
- [Google Scholar]
- Vertebral artery-posteroinferior cerebellar artery aneurysms: clinical and lower cranial nerve outcomes in 52 patients. Neurosurgery. 2005;56(1):2-10.
- [Google Scholar]
- Far lateral suboccipital approach for the treatment of proximal posteroinferior cerebellar artery aneurysms: surgical results and long-term outcome. Neurosurgery. 2004;55(1):39-50, discussion50–54.
- [Google Scholar]
- Surgical management of fusiform aneurysms of the peripheral posteroinferior cerebellar artery. Neurosurgery. 2003;53(4):831-834.
- [Google Scholar]
- Surgery on a saccular vertebral artery-posterior inferior cerebellar artery aneurysm via the transcondylar fossa (supracondylar transjugular tubercle) approach or the transcondylar approach: surgical results and indications for using two different lateral skull base approaches. J Neurosurg. 2001;95(2):268-274.
- [Google Scholar]
- Posteroinferior cerebellar artery aneurysms: surgical results for 38 patients. Neurosurgery. 1998;43(5):1026-1032.
- [Google Scholar]
- Management of aneurysms of the vertebral artery-posterior inferior cerebellar artery complex. Neurol Med Chir (Tokyo). 1998;38(08):93-103.
- [Google Scholar]
- Classification and treatment of vertebral dissecting aneurysm. Surg Neurol. 1997;48(6):598-605.
- [Google Scholar]
- Clinical analysis of a series of vertebral aneurysm cases. Neurosurgery. 1992;31(6):987-993, discussion 993.
- [Google Scholar]
- Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990;72(2):183-188.
- [Google Scholar]
- Peripheral aneurysms of the cerebellar arteries. Review of 16 cases. J Neurosurg. 1983;58(1):63-68.
- [Google Scholar]
- Multimodality treatment of posterior inferior cerebellar artery aneurysms. World Neurosurg. 2017;106:493-503.
- [CrossRef] [Google Scholar]
- Aneurysms with persistent patency after treatment with the pipeline embolization device. J Neurosurg. 2017;126(6):1894-1898.
- [Google Scholar]
- Endovascular coiling of ruptured very small dissecting fusiform aneurysm of posterior inferior cerebellar artery with parent artery preservation by microcatheter auto-assistance. World Neurosurg. 2019;121:152-155.
- [Google Scholar]
- Intracranial-to-intracranial bypass for posterior inferior cerebellar artery aneurysms: options, technical challenges, and results in 35 patients. J Neurosurg. 2016;124(5):1275-1286.
- [Google Scholar]
- Surgically managed pediatric intracranial aneurysms: how different are they from adult intracranial aneurysms? Pediatr Neurosurg. 2017;52(5):313-317.
- [Google Scholar]
- Treatment and outcomes among 102 posterior inferior cerebellar artery aneurysms: a comparison of endovascular and microsurgical clip ligation. World Neurosurg. 2015;83(5):784-793.
- [Google Scholar]
- Management of posterior inferior cerebellar artery aneurysms: what factors play the most important role in outcome? Acta Neurochir (Wien). 2017;159(3):549-558.
- [Google Scholar]
- Safety and efficacy of treatment strategies for posterior inferior cerebellar artery aneurysms: a systematic review and meta-analysis. Acta Neurochir (Wien). 2016;158(12):2415-2428.
- [Google Scholar]






