Translate this page into:
Endoscopic Intraventricular Atrial Adhesiolysis for the Treatment of Entrapped Temporal Horn after İntraventricular Tumor Surgery
Bashar Abuzayed, MD Department Neurosurgery, Gardens Hospital Al Sab Bin Jathamah St 20, P.O. Box 930186, Amman Jordan sylvius@live.com
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
A 21-year-old male patient was operated for third ventricle tumor (central neurocytoma) and showed improvement in the early postoperative period. After 2 weeks of surgery, the patient neurologic status deteriorated with acute decreased level of consciousness. Neuroimaging of the brain revealed entrapped left temporo-occipital horns with adhesion bands at the level of the atrium. Patient was operated with neuronavigation-guided endoscopic approach to the left atrium through the left posterior parietal region. Band adhesiolysis was performed with no complications. The patient showed fast improvement, and follow-up brain magnetic resonance imaging after 2 years showed the release of the ventricular entrapment with significant regression of the left ventricle size.
Keywords
adhesiolysis
neuroendoscope
trapped ventricle
Introduction
Entrapped temporal horn is a unique form of obstructive hydrocephalus secondary to an obstruction at the level of the trigone of the lateral ventricle. Continuous cerebrospinal fluid (CSF) production from the choroid plexus in the temporal horn with the obstruction of its outflow results in progressive dilation of the temporal horn. Trapped temporal horn syndrome—homonymous hemianopia due to compression of Meyer’s loop, hemiparesis due to internal capsule compression, memory disturbances due to compression of the hippocampus, and the signs of increased intracranial pressure—when develops, requires surgical intervention.1 Historically, ventriculoperitoneal shunt was the treatment of choice for this pathology.2 Other treatment options were described, such as resection of the temporal tip, temporal horn ventriculocisternostomy, temporal to frontal horn shunt, and temporal to prepontine cistern shunt.1 2 3 4
In this study, we present a case with entrapped temporal horn developed after intraventricular tumor resection due to adhesions in the resection bed, and treated with successful short- and long-term results with endoscopic adhesiolysis.
Case Report
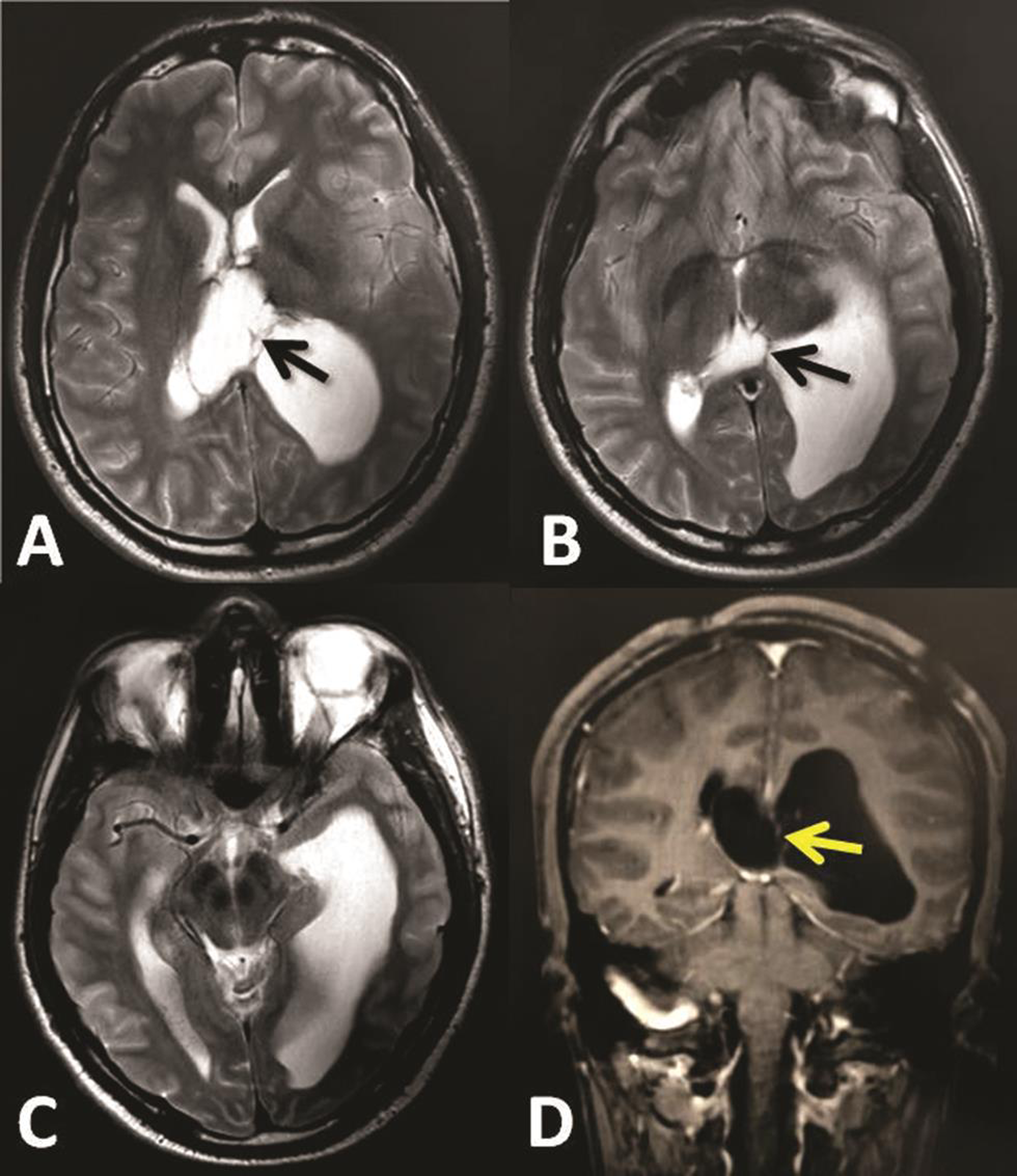
A 21-year-old male patient presented with acute headache, vomiting, and drowsiness. Neurological examination revealed Glasgow coma score (GCS) of 14/15 with no other neurologic deficit. Brain computed tomography (CT) scan and magnetic resonance imaging (MRI) showed central third ventricular mass lesion extending to the left lateral ventricle and left atrium and causing isolated left ventriculomegaly (Fig. 1). Patient was operated by interhemispheric transcallosal approach to remove the tumor totally and histopathology revealed the diagnosis of central neurocytoma. Early postoperative period was uneventful and patient showed improvement and was discharged after 5 days. One week later, the patient referred to our emergency service complaining vomiting and acute decrease in his level of the consciousness. GCS was 12/15 and pupils were equal and reactive. Emergency brain CT scan and MRI revealed entrapped left temporo-occipital horns with adhesion bands at the level of the atrium with no residual tumor (Fig. 2).

-
Fig. 1 (A–D) First preoperative brain magnetic resonance imaging showing central third ventricular tumor extending to the left lateral ventricle and causing isolated left ventriculomegaly.
Fig. 1 (A–D) First preoperative brain magnetic resonance imaging showing central third ventricular tumor extending to the left lateral ventricle and causing isolated left ventriculomegaly.
-
Fig. 2 (A–D) Brain magnetic resonance imaging 2 weeks after tumor removal, showing revealed trapped left temporo-occipital horns with adhesion bands (arrows) in the level of the atrium.
Fig. 2 (A–D) Brain magnetic resonance imaging 2 weeks after tumor removal, showing revealed trapped left temporo-occipital horns with adhesion bands (arrows) in the level of the atrium.
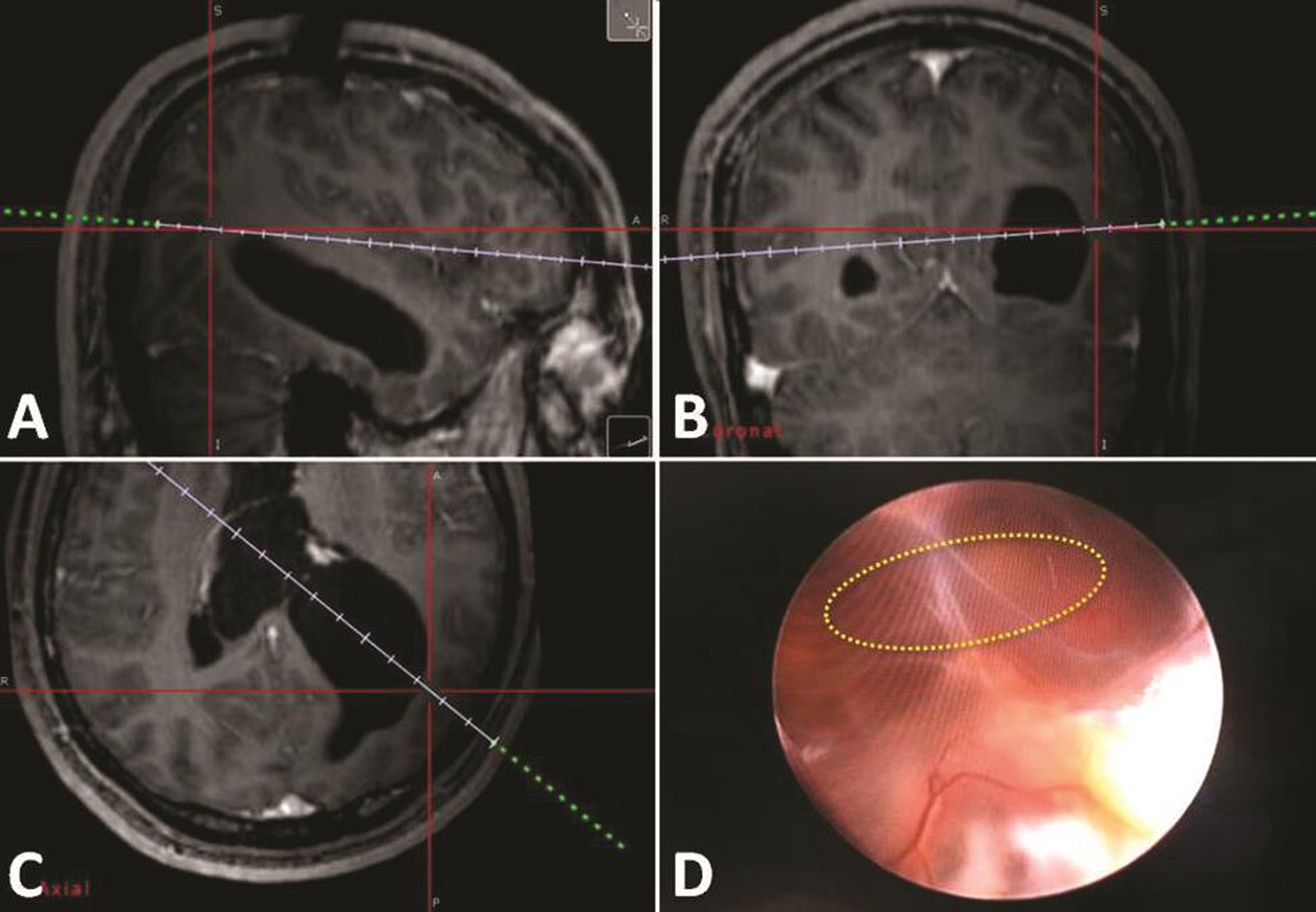
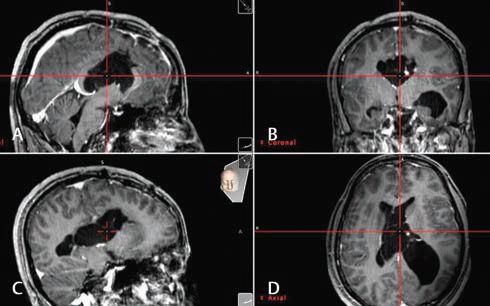
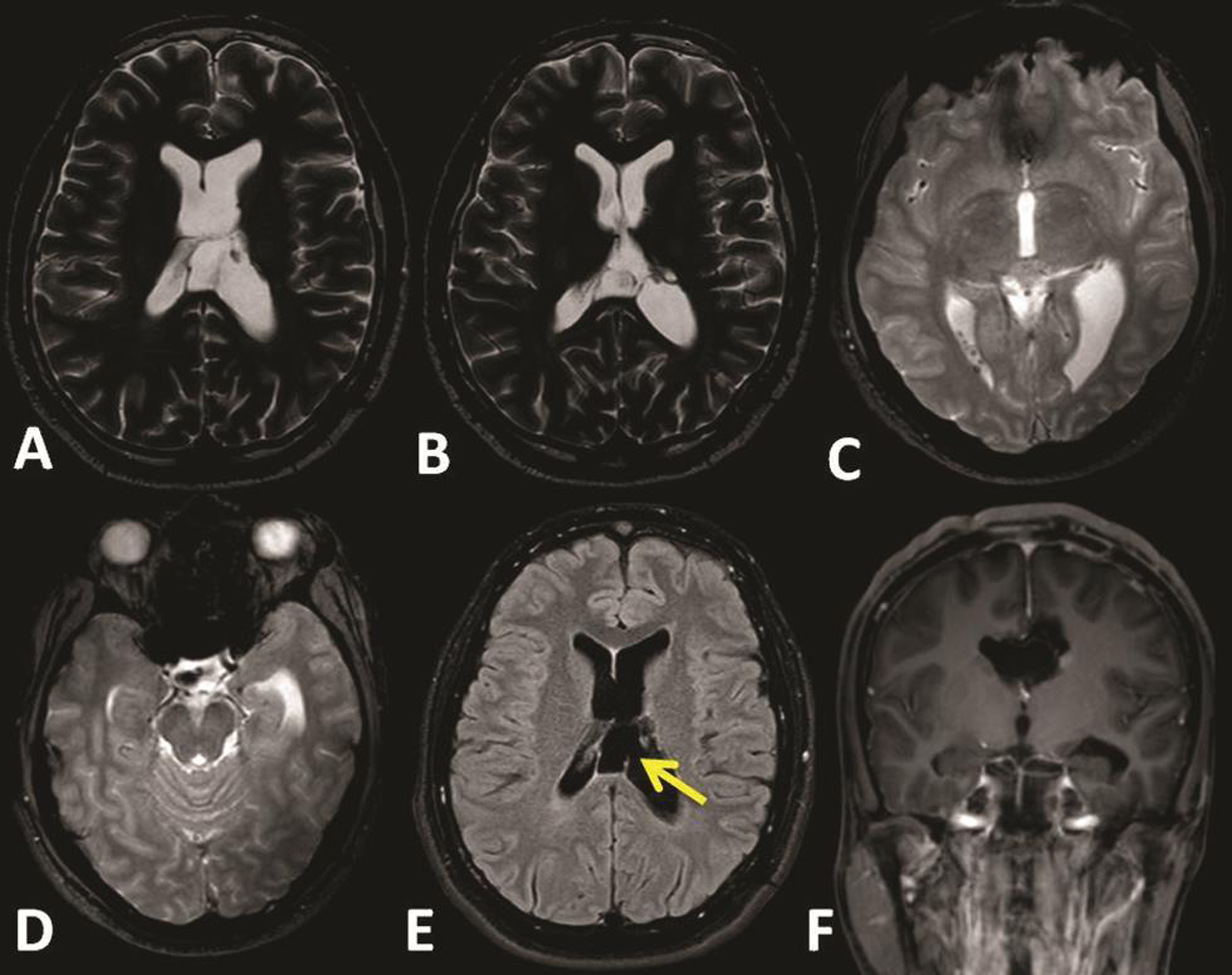
The patient was operated in supine position with full tilt of the head toward the right side. Using the neuronavigation guidance, the entry point burr hole was planned upon the left posterior parietal region to target the left atrium. This was similar to the planning for atrium approach with microsurgical technique. We used 0 degree Little LOTTA rigid endoscope system (Karl Storz, Tuttlingen, Germany), which is the same endoscope used for third ventriculostomy. Neuronavigation-guided neuroendoscope insertion into the occipital horn and reaching the adhesion band was performed (Fig. 3). Adhesiolysis was performed first by puncturing the band, then dilation of the stoma with 2 mm Fogarty catheter. At the end, the endoscope was introduced into the body of the left ventricle and the posterior part of the left temporal, exploration of these spaces was performed, and surgery was then ended (Fig. 4). After surgery, no complications were seen and patient showed fast improvement and was discharged after 4 days. Follow-up brain MRI after 2 years showed no tumor recurrence and the release of the ventricular entrapment with significant regression of the left ventricle size (Fig. 5).
-
Fig. 3 (A–C) Intraoperative neuronavigation-guided planning for the entry point burr hole and the trajectory of the endoscopic approach. (D) Intraoperative endoscopic view of the adhesion band and the site of adhesiolysis (circle).
Fig. 3 (A–C) Intraoperative neuronavigation-guided planning for the entry point burr hole and the trajectory of the endoscopic approach. (D) Intraoperative endoscopic view of the adhesion band and the site of adhesiolysis (circle).
-
Fig. 4 (A–D) Intraoperative neuronavigation views after adhesiolysis and more advancing of the endoscope toward the body of the left ventricle. The cross point shows the real-time location of the tip of the endoscope.
Fig. 4 (A–D) Intraoperative neuronavigation views after adhesiolysis and more advancing of the endoscope toward the body of the left ventricle. The cross point shows the real-time location of the tip of the endoscope.
-
Fig. 5 (A–F) Follow-up brain magnetic resonance imaging after 2 years of surgery, showing no tumor recurrence, the fenestration site (arrow), and the release of the ventricular entrapment with significant regression of the left ventricle size.
Fig. 5 (A–F) Follow-up brain magnetic resonance imaging after 2 years of surgery, showing no tumor recurrence, the fenestration site (arrow), and the release of the ventricular entrapment with significant regression of the left ventricle size.
Discussion
Temporal horn entrapment was first described by Cairns and Daniel in 1947 as a form of noncommunicating focal hydrocephalus.5 It can develop secondary to penetrating trauma, meningitis, ventriculitis, intraventricular abscess, intraventricular hemorrhage, and intraventricular arachnoid cysts.2 3 CSF pathway obstruction due to adhesions after tumor resection, such as our case, is a rarely reported etiology.1 4
Ventriculoperitoneal shunting is being the most common used method for the treatment of entrapped temporal horn.2 Neuroendoscopic surgery is an effective technique to treat obstructive hydrocephalus by direct fenestration of the blocked site in the CSF pathway or by bypassing the obstruction site with fenestration into the ventricles or subarachnoid spaces.2 Few reported cases described the treatment of entrapped temporal horn with endoscopic temporal ventriculocisternostomy.1 3 Although there is no standard guideline for the treatment method for these situations, there is general acceptance that attempts should be directed to reconnect the CSF pathway whenever possible, either by restoration of the original path to the atrium or by creating a communication to the subarachnoid space with opening the choroidal fissure.2
Although endoscopic temporal ventriculocisternostomy showed to be safe and effective technique with good results in the reported cases, some studies concluded that this technique is not risk free of complications. Hasegawa et al reported two cases of postinfectious isolated temporal ventriculomegaly treated with endoscopic temporal ventriculocisternostomy.1 One case suffered from cerebral infarction due to injury of the branches of the choroidal segment of the anterior choroidal artery. They stated that it is still a risky technique, and anatomic knowledge and surgical experience required before it can be recommended.1
For the diagnosis, determination of the obstruction site and surgical planning, MRI with routine T1- and T2-weighted images obtained in the axial, coronal, and sagittal planes are performed. Moreover, thin membranes that might interfere with CSF circulation can be detected with constructive interference in steady state sequences are performed to detect. CSF flow can be studied with T2-weighted sagittal inversion recovery turbo spin echo and cine phase contrast MRI.2 Also, the pathoanatomic distortion of the ventricles with the known previous medical history of the patient can be indicators to the site of the obstruction. In our case, beside the radiologic evidence of the adhesion bands, the site of the volume transition from normal ventricle body to dilated occipitotemporal horns gave a clue about the site of the obstruction, which was the atrium.
Many studies addressed the site of the burr hole for the entry point to access the occipital horn. Schroeder et al described the entry point just superior and posterior to the ear in the temporo-occipital region,2 thus, forming a straight trajectory from the entry point to the tip of the temporal horn. In an anatomic and radiologic morphometric study by Sánchez et al, they found that the mean entry point was 2.74 cm lateral and 5.65 cm superior to the inion.4 From this entry point, the mean trajectory angulations were 5.12 degrees to the middle sagittal plane and 30.92 degrees to the orbitomeatal plane.4 These studies mainly aimed to enter the occipital, atrium, temporal tract, thus, their entry point was more medial toward midline than our approach. Our approach was aimed to enter the body of the lateral ventricle. This needed more lateral entry point and more medially angulated trajectory. Thus, our approach was planned more similar to microsurgical approach to the atrium.
In our case, after exposure of the adhesion band, adhesiolysis was performed first by puncturing the band, then dilation of the stoma with 2 mm Fogarty catheter. Both the body of the left ventricle and the posterior part of the left temporal horn were entered and exposed, although feasible surgical fenestration of scar has possible risk of rescarring and reobstruction.1 Some studies reported the stent use to support the stoma and prevent reclosure.3 Marx et al described neuroendoscopic stenting for CSF pathway obstructions in adults.3 Patients had arachnoid cysts, foramen of Monro or aqueduct stenosis, and CSF obstruction secondary to tumors. Results showed no mortalities or infections; however, late stent dislocation or migration was seen in three patients (12%). They stated that it is a good option in cases of CSF pathway obstruction secondary to tumors to avoid reclosure by tumor growth.3 In our case, we performed fenestration and dilation of the scar without stenting. This technique showed to be efficient in our case, as long-term results after 2 years showed good clinical and radiological outcome.
Conclusion
Endoscopic intraventricular adhesiolysis is an effective and simple technique for treating entrapped temporal horn after intraventricular tumor surgery. It can be an alternative for shunting or endoscopic temporal ventriculocisternostomy in carefully selected cases. More case studies with long-term follow-up are required to evaluate the efficiency technique for more wide application in general practice.
Conflict of Interest
None declared.
References
- Risks of endoscopic temporal ventriculocisternostomy for isolated lateral ventricle: anatomic surgical nuances. World Neurosurg. 2018;110:189-192.
- [Google Scholar]
- Endoscopic treatment of cerebrospinal fluid pathway obstructions. Neurosurgery. 2008;62(6)(03):1084-1092.
- [Google Scholar]
- Neuroendoscopic stent placement for cerebrospinal fluid pathway obstructions in adults. J Neurosurg. 2016;125(3):576-584.
- [Google Scholar]
- New endoscopic route to the temporal horn of the lateral ventricle: surgical simulation and morphometric assessment. J Neurosurg. 2014;121(3):751-759.
- [Google Scholar]
- Localized hydrocephalus following penetrating wounds of the ventricle. Br J Surg. 1947;55(01):187-197.
- [Google Scholar]






