Translate this page into:
Profile of Scrub Typhus Meningitis/Meningoencephalitis in Children with and without Scrub Typhus IgM Antibody in CSF
Rashmi Ranjan Das, MD Department of Pediatrics, All India Institute of Medical Sciences Bhubaneswar, Odisha 751019 India rrdas05@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The aim of this article was to study the spectrum of scrub typhus meningitis/meningoencephalitis (STM) cases in children. Children ≤14 years of age with acute undifferentiated febrile illness were included. Immunoglobulin M (IgM) enzyme-linked immunosorbent assay was done in blood and cerebrospinal fluid (CSF) of children with suspected STM. Demographic, clinical, and laboratory details were expressed as descriptive statistics. Factors associated with neurological involvement were identified on univariate analysis. A total of 76 children had ST during the study period (meningitis/meningoencephalitis = 8 [10.5%], of which 5 [62.5%] had detectable ST IgM antibodies in CSF). The included children were 4 to 12 years of age with boys > girls. Headache and vomiting were common in those with STM, whereas hyponatremia and thrombocytopenia were common in those without STM. All children with STM recovered with sequelae in one child (right lateral rectus palsy). There was no mortality. STM has an incidence of 10.5% in children with ST from Eastern India. Headache and vomiting were significant predictors of STM, whereas hyponatremia and thrombocytopenia were significant predictor of non-STM.
Keywords
children
meningitis
meningoencephalitis
scrub typhus
Orientia tsutsugamushi
Introduction
Scrub typhus (ST), caused by a mite-borne bacteria Orientia tsutsugamushi, is one of the reemerging infectious diseases in the Indian subcontinent.1 This resurgence in cases of ST is attributed to deforestation, antimicrobial prescription practices, and availability of better diagnostic modalities.2 The manifestations range from asymptomatic illnesses to severe forms resulting in multiorgan dysfunction syndrome and death.3 4 Pediatric scrub typhus meningitis/meningoencephalitis (STM) incidence varies from 6 to 30% across various studies published from tropical countries.5 6 7 8 9 10 11 12 However, none of these studies have tried to detect ST in cerebrospinal fluid (CSF) specimen. Instead, they have used blood immunoglobulin M (IgM) positivity as a surrogate marker of STM in a compatible clinical setting (features of meningitis/encephalitis). The current study attempted to describe the profile and outcome of STM children with and without ST IgM antibody in CSF.
Methods
This cross-sectional study was spanned over 18 months period (January 2017 to June 2018) in the pediatrics department of a tertiary care teaching hospital from Eastern part of India. Children ≤14 years of age with acute undifferentiated febrile illness (AUFI) were included.13 Enrolment of eligible children was done after obtaining consent from parents/legal guardian. Approval of Institute's Ethics Committee (IMF/04/2016) was obtained before start of the study.
Laboratory tests including complete blood count, serum electrolytes, liver, and renal function tests were done in all the patients. Microbiological analysis included blood culture, peripheral smear examination for malaria parasite and malarial antigen detection test, dengue enzyme-linked immunosorbent assay (ELISA) NS1 antigen and IgM capture ELISA, Widal test, and IgM ELISA against Orientia tsutsugamushi (InBios International, Inc., Seattle, Washington, United States). Children having features of meningitis/meningoencephalitis (headache, altered sensorium, neck rigidity, seizure, or focal neurological deficit) underwent CSF analysis. CSF (diluted in 1:10 proportion) of laboratory-confirmed ST cases were further tested by ELISA for antibody (IgM) against Orientia tsutsugamushi. Optical density values ≥1.00 and ≥0.5 were taken as cutoffs from serum and CSF, respectively. Either doxycycline or azithromycin was used as treatment for duration of 10 days.
Statistical Analysis
Data were analyzed after entering them into Microsoft Excel spreadsheet followed by application of STATA software version 12.0 (College Station, Texas, United States). Demographic, clinical, and laboratory details were expressed as number (%), mean (standard deviation) or median (interquartile range). Student's t-test (for normally distributed data) or Mann–Whitney U test (for skewed data) was used to analyze continuous data. Fisher's exact test or chi-squared test was used to analyze categorical data. For identification of factors associated with neurological involvement, univariate analysis was performed. Statistical significance was set at a p-value of <0.05.
Results
A total of 171 children with undifferentiated fever (AUFI) were admitted, and 76 (44.4%) had positive blood IgM antibody against Orientia tsutsugamushi (Fig. 1). Of them, eight children (10.5%) were found to have meningitis/meningoencephalitis (STM); five (62.5%) children were CSF positive for ST IgM antibodies. The age of included children ranged from 4 to 12 years, and boys were commonly affected (3 times more than girls).
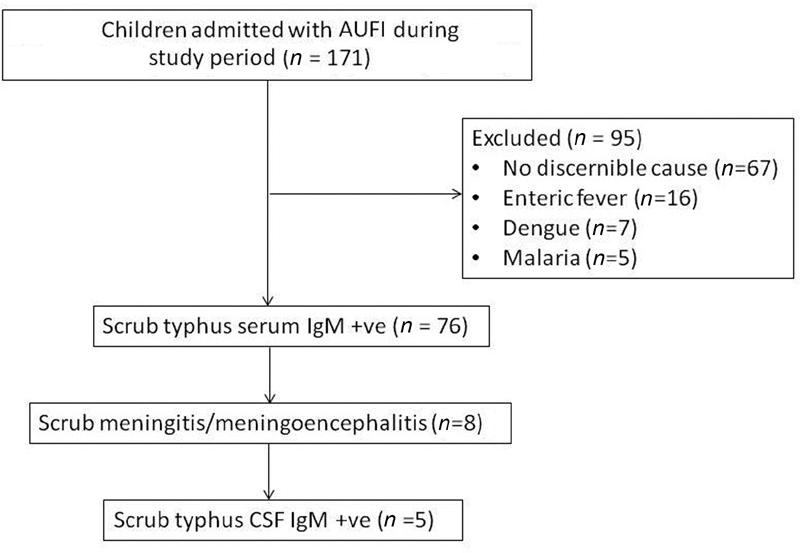
-
Fig. 1 Flowchart of study children. AUFI, acute undifferentiated febrile illness; CSF, cerebrospinal fluid; IgM, immunoglobulin M.
Fig. 1 Flowchart of study children. AUFI, acute undifferentiated febrile illness; CSF, cerebrospinal fluid; IgM, immunoglobulin M.
The clinical and laboratory findings, treatment, and outcome details have been described in Tables 1 and 2. All the children recovered with sequelae in one child (right lateral rectus palsy). No child required intensive care unit admission.
|
Characteristics |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
Patient 6 |
Patient 7 |
Patient 8 |
|---|---|---|---|---|---|---|---|---|
|
Age (y)/sex |
9 / M |
8 / M |
4 / M |
8 / F |
11 / M |
9 / M |
12 / M |
12 / F |
|
Fever duration (d) |
7 |
5 |
4 |
8 |
3 |
7 |
4 |
5 |
|
Chills and rigor |
No |
Yes |
Yes |
No |
No |
No |
No |
No |
|
Others |
No |
Pain abdomen, vomiting |
Abdomen distension, vomiting |
Pain abdomen, vomiting |
Vomiting |
Reeling of head |
Photophobia, anorexia |
Dizziness |
|
Seizure |
Yes |
No |
No |
No |
No |
Yes |
No |
No |
|
Headache |
No |
Yes |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Ataxia |
No |
No |
Yes |
No |
No |
No |
No |
No |
|
Altered sensorium |
Yes |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
|
Meningeal sign |
No |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
|
Tachycardia |
No |
No |
No |
No |
Yes |
No |
No |
No |
|
Low BP |
No |
No |
No |
No |
Yes |
No |
No |
No |
|
Tachypnea |
Yes |
No |
Yes |
No |
Yes |
No |
No |
No |
|
Rash (nonpruritic) |
No |
Yes |
No |
No |
No |
No |
Yes |
No |
|
Eschar |
No |
No |
No |
Yes |
No |
No |
No |
No |
|
Pallor |
No |
Yes |
Yes |
No |
No |
No |
Yes |
No |
|
Edema |
No |
No |
Yes |
Yes |
No |
No |
No |
No |
|
Icterus |
No |
No |
No |
Yes |
No |
No |
No |
No |
|
Lymphadenopathy |
No |
No |
Inguinal nodes (tender) |
Neck nodes |
No |
No |
No |
No |
|
Hepatomegaly |
No |
Yes |
Yes |
Yes |
No |
No |
Yes |
Yes |
|
Splenomegaly |
No |
Yes |
No |
No |
No |
No |
Yes |
No |
Abbreviations: BP, blood pressure; F, female; M, male.
|
Findings |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
Patient 6 |
Patient 7 |
Patient 8 |
|---|---|---|---|---|---|---|---|---|
|
Hemoglobin (g/dL) |
10.1 |
9.8 |
9.6 |
9.9 |
12.7 |
11.1 |
10.3 |
11.5 |
|
White blood cell count (cells/mm3) |
12,950 |
4,810 |
17,810 |
18,570 |
8,790 |
6,810 |
5720 |
7890 |
|
Platelet count (lakhs/mm3) |
2.24 |
1.32 |
2.49 |
1.2 |
1.72 |
2.49 |
1.04 |
1.54 |
|
ESR (mm/h) |
36 |
28 |
30 |
53 |
42 |
26 |
42 |
24 |
|
Serum bilirubin (mg/dL): total/direct |
0.4/0.1 |
1.4/0.5 |
0.2/0.1 |
0.7/0.3 |
0.6/0.2 |
0.6/0.1 |
0.7/0.2 |
0.5/0.1 |
|
AST (IU/L) |
36 |
67 |
87 |
107 |
55 |
111 |
161 |
64 |
|
ALT (IU/L) |
43 |
53 |
44 |
50 |
95 |
42 |
58 |
34 |
|
ALP (IU/L) |
136 |
182 |
224 |
187 |
152 |
212 |
241 |
252 |
|
Total protein (g/dL) |
7.8 |
5.4 |
5.6 |
6.4 |
6.8 |
5.9 |
5.8 |
6.4 |
|
Albumin (g/dL) |
3.2 (low) |
2.9 |
2.6 |
2.4 |
3.5 |
2.7 |
2.6 |
3.1 |
|
Urea (mg/dL) |
24 |
35 |
28 |
29 |
21 |
28 |
29 |
21 |
|
Creatine (mg/dL) |
0.5 |
0.6 |
0.5 |
0.4 |
0.8 |
0.4 |
0.6 |
0.4 |
|
Sodium (mmol/L) |
128 |
130 |
137 |
123 |
133 |
131 |
126 |
132 |
|
Potassium (mmol/L) |
4.9 |
3.7 |
4.3 |
4.6 |
3.9 |
4.1 |
4.7 |
3.8 |
|
Glucose (mg/dL), CSF |
20 |
55 |
67 |
54 |
62 |
72 |
66 |
58 |
|
Protein (mg/dL), CSF |
73 |
56 |
192 |
55 |
27 |
44 |
36 |
60 |
|
Cytology/mm3 (% lymphocytes), CSF |
12 (100) |
24 (60) |
102 (68%) |
6 (100) |
10 (99) |
13 (72) |
10 (100) |
16 (77) |
|
Scrub typhus CSF IgM ELISA |
+ve |
+ve |
+ve |
+ve |
+ve |
−ve |
−ve |
−ve |
|
Scrub typhus serum IgM ELISA |
+ve |
+ve |
+ve |
+ve |
+ve |
+ve |
+ve |
+ve |
|
CT brain |
Normal |
– |
Edema |
– |
– |
– |
– |
– |
|
Antibiotics given for ST |
Azithromycin |
Azithromycin (+ ceftriaxone) |
Azithromycin (+ ceftriaxone) |
Azithromycin |
Azithromycin (+ ceftriaxone) |
Azithromycin (+ ceftriaxone) |
Doxycycline |
Doxycycline (+ ceftriaxone) |
|
Outcome |
Recovered |
Recovered |
Right lateral rectus palsy |
Recovered |
Recovered |
Recovered |
Recovered |
Recovered |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CSF, cerebrospinal fluid; CT, computed tomography; ELISA, enzyme-linked immunosorbent assay; ESR, erythrocyte sedimentation rate; IgM, immunoglobulin M.
On univariate analysis, headache and vomiting emerged as significant predictors of STM, whereas thrombocytopenia and hyponatremia were significant predictors of non-STM (Table 3).
|
Clinical and laboratory predictors |
Total number of cases (n = 76) |
Odds ratio (OR) |
95% confidence interval (CI) |
p-Value |
|
|---|---|---|---|---|---|
|
Without CNS involvement (n = 68) |
With CNS involvement (n = 8) |
||||
|
Clinical predictors |
|||||
|
Age (y) (mean ± SD) |
8.6 ± 3.7 |
9.1 ± 2.6 |
– |
– |
0.26 |
|
Male, n (%) |
41 (60.3) |
06 (75) |
1.98 |
0.37–10.52 |
0.41 |
|
Headache, n (%) |
19 (27.9) |
05 (62.5) |
5.0 |
1.08–23.16 |
0.021a |
|
Vomiting, n (%) |
12 (17.6) |
04 (50) |
4.67 |
1.02–21.33 |
0.034a |
|
Pain abdomen, n (%) |
21 (30.1) |
02 (25) |
0.75 |
0.14–4.01 |
0.73 |
|
Rash (nonpruritic), n (%) |
07 (10.3) |
02 (25) |
2.9 |
0.49–17.25 |
0.22 |
|
Eschar, n (%) |
03 (4.4) |
01 (12.5) |
3.1 |
0.28–33.91 |
0.33 |
|
Pallor |
21 (30.1) |
03 (37.7) |
1.34 |
0.29–6.15 |
0.71 |
|
Tachycardia |
29 (42.6) |
01 (12.5) |
0.19 |
1.02–1.65 |
0.09 |
|
Hypotension |
13 (19.1) |
01 (12.5) |
0.6 |
0.07–5.35 |
0.64 |
|
Icterus |
06 (8.8) |
01 (12.5) |
1.48 |
0.15–14.1 |
0.74 |
|
Hepatosplenomegaly |
23 (34.2) |
01 (12.5) |
0.65 |
0.12–3.49 |
0.61 |
|
Laboratory predictors |
|||||
|
Hemoglobin (g/dL) (mean ± SD) |
9.78 ± 1.73 |
9.52 ± 1.62 |
– |
– |
0.37 |
|
Total leucocyte count (×109 cells/L) (median (IQR)) |
11.1 (3.1–20.6) |
8.3 (4.7–12.2) |
– |
– |
0.54 |
|
Platelet counts (×109/L) (median (IQR)) |
0.87 (0.45–3.23) |
1.33 (1.2–2.18) |
– |
– |
0.046a |
|
Urea (mg/dL) (mean ± SD) |
24.7 ± 10.42 |
21.5 ± 9.1 |
– |
– |
0.39 |
|
Creatine (mg/dL) (mean ± SD) |
0.9 ± 0.42 |
0.5 ± 0.1 |
– |
– |
0.43 |
|
Sodium (mEq/L) (mean ± SD) |
124.6 ± 4.7 |
132.7 ± 3.9 |
– |
– |
0.012a |
|
Serum bilirubin (mg/dL) (mean ± SD) |
1.08 ± 0.9 |
0.8 ± 0.4 |
– |
– |
0.28 |
|
AST (IU/L), mean ± SD |
86.9 ± 53.5 |
74.3 ± 38.7 |
– |
– |
0.13 |
|
ALT (IU/L), mean ± SD |
51.6 ± 22.2 |
49.1 ± 19.5 |
– |
– |
0.56 |
|
Total serum protein (g/dL), mean ± SD |
7.7 ± 2.64 |
8.1 ± 1.4 |
– |
– |
0.47 |
|
Serum albumin (g/dL), mean ± SD |
2.9 ± 1.04 |
3.1 ± 1.1 |
– |
– |
0.35 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CI, confidence interval; CNS, central nervous system; IQR, interquartile range; OR, odds ratio; SD, standard deviation.
Discussion
ST was commonest cause of AUFI in children (44.4%) who presented during the study period. Meningitis/meningoencephalitis was seen in 10.5% children, which is in accordance with previously published studies.5 6 7 8 9 10 11 12 All the children recovered with sequelae in one child (right lateral rectus palsy). Headache and vomiting emerged as significant predictors of STM, whereas thrombocytopenia and hyponatremia were significant predictors of non-STM.
ST is a multisystem infectious vasculitis that can cause a variety of neurological manifestations. The mechanisms of brain invasion by the organism include infection of monocytes and vascular invasion.5 However, recent reports have also highlighted direct invasion of brain by the organism.14 15 One study analyzed the rickettsial DNA in CSF of 25 patients with meningitis by means of nested polymerase chain reaction (PCR).14 The authors noted mild pleocytosis in 48% (from 0 to 110/mm3 with mean mononuclear cell count of 52%), increased protein level (>50 mg/dL) in 28%, and a positivity nested PCR in 24% patients. Based on their findings, the authors suggested that Orientia tsutsugamushi causes CSF invasion, and in endemic areas, it should be considered in the differential diagnoses of mononuclear meningitis. In the present study, we found the cell count ranging from 6 to 102/mm3 (60–100% mononuclear cells), and the protein was elevated in 75% of children. Our findings were similar to other studies published in children from other parts of India.5 7
Of various clinical features of meningitis, headache was noted in 62.5% and vomiting in 50% patients. Other pediatric studies have found headache in <50% children, though headache is seen in >60% of adults with STM.16 However, vomiting frequency noted in the current study is similar to other studies in children.5 7 In the current study, seizure was seen in 25% cases that was similar to other studies,7 but lower than another Indian study.5 However, the frequency of altered sensorium (62.5%) was similar to the later study.5 Eschar is a pathognomonic feature, and is seen with variable frequencies in children with STM. Present study noted it in 12.5% children in contrast to other studies where it was reported in >20% children.5 7 However, eschar was more commonly found in children with STM compared with those without STM (4.4%) in the present study. Other features of central nervous system involvement in the present study included ataxia and right lateral rectus palsy (6th cranial nerve palsy). Three children had features of raised intracranial pressure with papilledema, and computed tomography scan of brain performed in two children showed cerebral edema in one. All these are similar to a published study from North India.17 Azithromycin was the mainstay of treatment in the present study (75%), in contrast to doxycycline (25%) used in all previous studies. However, all of our children recovered completely without any mortality. This is an interesting finding that needs further confirmation in a clinical trial. Azithromycin has a better safety profile in children compared with doxycycline.
When we analyzed the predictors of STM, we found headache and vomiting to be significant predictors of STM, whereas hyponatremia and thrombocytopenia were significant predictors of non-STM. In a previous study, hemoglobin level and total platelet counts, compared with meningitis group, were significantly lower in no meningitis group.7 The reason for this may be because of a longer duration of illness in those without meningitis with a greater number of sick cases. However, none of the previous studies have found headache and vomiting to be significant predictors of STM.
The strength of the present study is that we used CSF IgM antibody positivity to confirm STM cases. However, there are some limitations. First, IFA (indirect fluorescent antibody) test is considered as the gold standard,18 but IgM ELISA test was used in the current study. Second, we could not carry out PCR to isolate Orientia tsutsugamushi from CSF and differentiate its diverse serotypes.
Conclusions
In the current study, meningitis/meningoencephalitis was noted in 10.5% of children with STM. Headache and vomiting were significant predictors of STM, whereas hyponatremia and thrombocytopenia were significant predictors of non-STM.
Conflict of Interest
None declared.
Funding The study was funded by institute intramural grant of AIIMS Bhubaneswar. The grant is provided to junior faculties to promote research at the Institute.
References
- Scrub typhus re-emergence in India: Contributing factors and way forward. Med Hypotheses. 2018;115:61-64.
- [Google Scholar]
- Scrub typhus: risks, diagnostic issues, and management challenges. Res Rep Trop Med. 2017;8:73-83.
- [Google Scholar]
- Clinico-epidemiological analysis of scrub typhus in hospitalised patients presenting with acute undifferentiated febrile illness: a hospital-based study from Eastern India. Indian J Med Microbiol. 2019;37(2):278-280.
- [Google Scholar]
- Scrub typhus: a clinico-laboratory differentiation of children with and without meningitis. J Trop Pediatr. 2016;62(3):194-199.
- [Google Scholar]
- CNS manifestations in Orientia tsutsugamushi disease (scrub typhus) in North India. Indian J Pediatr. 2016;83(7):634-639.
- [Google Scholar]
- Comparison of scrub typhus with and without meningitis. Indian J Pediatr. 2017;84(11):833-837.
- [Google Scholar]
- Comparison of scrub typhus meningitis with acute bacterial meningitis and tuberculous meningitis. Indian Pediatr. 2018;55(1):35-37.
- [Google Scholar]
- Clinical profile, complications and outcome of scrub typhus in children: a hospital based observational study in central Nepal. PLoS One. 2019;14(8):e0220905.
- [Google Scholar]
- Clinical profile of scrub typhus in children. Indian J Pediatr. 2012;79(11):1459-1462.
- [Google Scholar]
- Clinical profile and therapeutic response of scrub typhus in children: a recent trend from Eastern India. J Trop Pediatr. 2019;65(2):139-146.
- [Google Scholar]
- Clinico-laboratory profile, complications and therapeutic outcome of scrub typhus in children. J Nepal Health Res Counc. 2020;18(2):282-287.
- [Google Scholar]
- Nonmalarial acute undifferentiated fever in a rural hospital in central India: diagnostic uncertainty and overtreatment with antimalarial agents. Am J Trop Med Hyg. 2008;78(3):393-399.
- [Google Scholar]
- Central nervous system involvement in patients with scrub typhus. Clin Infect Dis. 1997;24(3):436-440.
- [Google Scholar]
- Neuroinflammation associated with scrub typhus and spotted fever group rickettsioses. PLoS Negl Trop Dis. 2020;14(10):e0008675.
- [Google Scholar]
- Tsutsugamushi disease (scrub typhus) meningoencephalitis in North Eastern India: a prospective study. Ann Med Health Sci Res. 2015;5(3):163-167.
- [Google Scholar]
- Clinical profile and predictors of intensive care unit admission in pediatric scrub typhus: a retrospective observational study from North India. Indian J Crit Care Med. 2020;24(6):445-450.
- [Google Scholar]
- An ELISA assay using a combination of recombinant proteins from multiple strains of Orientia tsutsugamushi offers an accurate diagnosis for scrub typhus. BMC Infect Dis. 2017;17(1):413.
- [Google Scholar]







