Translate this page into:
Effect of Preanesthetic Fluid Loading on Postinduction Hypotension and Advanced Cardiac Parameters in Patients with Chronic Compressive Cervical Myelopathy: A Randomized Controlled Trial
Kamath Sriganesh, DM Department of Neuroanaesthesia and Neurocritical Care, Neurosciences Faculty Block, National Institute of Mental Health and Neurosciences Bengaluru 560076, Karnataka India drsri23@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Purpose Hypotension during the early intraoperative phase is common and can lead to adverse perioperative outcomes. Fluid preloading is one of the methods to limit its occurrence. Patients with chronic compressive cervical myelopathy may have autonomic dysfunction, which can aggravate hemodynamic alterations during anesthesia. This study compared the occurrence of postinduction hypotension and changes in cardiac dynamic indices in patients with and without crystalloid preloading undergoing decompressive cervical spine surgery.
Methods This randomized controlled trial was conducted over 15 months after obtaining patient consent, approval of the institute ethics committee, and trial registration. We compared preanesthetic fluid loading with Ringer's lactate (20 mL/kg over 30 minutes) with no preloading (2 mL/kg/h maintenance) in 60 consecutive patients undergoing cervical spine surgery. The ANSiscope was used to determine baseline cardiac autonomic function. Noninvasive cardiac output monitor was used to assess changes in heart rate, mean arterial pressure, cardiac index (CI), stroke volume variation (SVV), and total peripheral resistance index during study intervention, anesthetic induction, tracheal intubation, and change in position from supine to prone.
Results The incidences of postinduction hypotension were 26.7% (8/30) and 86.7% (26/30) and the median doses of mephentermine used were 0 and 6 mg, respectively, in patients with and without fluid preloading (both p < 0.001). Preloading resulted in improvement in CI, reduction in SVV, and lesser vasopressor use.
Conclusion Preloading reduced the occurrence of postinduction hypotension and vasopressor use, improved CI, and reduced SVV during the early intraoperative period.
Registration number of Clinical Trial The trial was registered with Clinical Trial Registry of India (CTRI/2018/07/014970 on 19/07/2018).
Keywords
fluid preloading
postinduction hypotension
dynamic cardiac indices
compressive cervical myelopathy
cardiac autonomic dysfunction
Introduction
The early intraoperative phase is a period starting from the induction of general anesthesia (GA) to the beginning of surgery. Hypotension is the most common adverse hemodynamic event in the early intraoperative period1 2 and is often associated with poor perioperative outcomes.3 4 5 Several factors predict the occurrence of postinduction hypotension.1 6 One of the measures used to minimize postinduction hypotension is preloading with intravenous fluids. The effect of preanesthetic fluid loading on ameliorating postinduction hypotension is equivocal, with some studies showing reduction in the incidence of hypotension with preanesthetic fluid loading,7 while others observing no beneficial effect.8 Therefore, for better understanding of postinduction hypotension, it is important to evaluate its mechanisms and assess the impact of fluid preloading by going beyond the routine blood pressure (BP) measurements and studying the advanced cardiac and hemodynamic parameters. A recent observational study in noncardiac patients demonstrated decrease in systemic vascular resistance (SVR) as a factor contributing to the development of postinduction hypotension.9 Another study showed that stroke volume variation (SVV) before anesthetic induction predicts postinduction hypotension.10 A decrease in cardiac index (CI) seen with induction agents such as propofol and etomidate also contributes to postinduction hypotension.11 Therefore, assessing the advanced hemodynamic parameters such as SVV, CI, and SVR is likely to provide greater insights into the cause of hypotension and also help evaluate the effect of fluid preloading on these variables.
Chronic compressive cervical myelopathy (CCCM) can affect the cardiac autonomic function. Patients with cardiac autonomic dysfunction (CAD) demonstrate an exaggerated hemodynamic response during anesthesia and surgery. It is currently unclear if fluid preloading can ameliorate the occurrence of postinduction hypotension in this population.
The primary objective of this study was to assess the effect of preanesthetic crystalloid preloading on the incidence of postinduction hypotension in patients undergoing decompressive surgery for CCCM. Our secondary objectives were to evaluate the effects of fluid loading on dynamic cardiac indices such as heart rate (HR), mean arterial pressure (MAP), SVV, CI, and total peripheral resistance index (TPRI) during the early intraoperative period.
Methods
Study Setting, Design, Ethics, and Trial Registration
This nonfunded, single-center, parallel-group randomized controlled trial (RCT) was conducted at the National Institute of Mental Health and Neurosciences (NIMHANS), Bengaluru, after approval of the institutional ethics committee [NIMHANS/IEC (BS & NS DIV.) 13th meeting/2018 held on June 20, 2018] and written informed consent from the patients or their legal guardian. The trial was registered with the Clinical Trial Registry of India (CTRI/2018/07/014970, registered on July 19, 2018).
Study Population and Period
All consecutive consenting eligible patients with CCCM who were scheduled for decompressive surgery during the 15-month period from August 2018 to October 2019 were recruited in the study. Patients were included if they had clinical symptoms of myelopathy of ≥3 months with imaging evidence, belonged to American Society of Anesthesiologists (ASA) physical status 1 and 2, and aged between 18 and 70 years. We excluded patients with atlantoaxial dislocation and cervicomedullary junction pathology, tumors, and preexisting systemic disease such as diabetes mellitus, hypertension, ischemic heart disease, and chronic obstructive pulmonary disease.
Control of Bias and Study Interventions
Randomization was done using computer-generated random number table with 1:1 allocation ratio. Study interventions were performed in the preoperative holding area using identical 500-mL fluid bottle (infusion rate determined as per randomization) by an individual not directly involved in the study. Thus, patient, anesthesiologist, outcome assessor, and data analyst were blinded to the group allocation. The patients in group F received 20 mL/kg of Ringer's lactate over 30 minutes, while those in group C received Ringer's lactate at the maintenance rate of 2 mL/kg/h.
Data Collection
Demographic details such as age, gender, and body mass index were collected. The noninvasive cardiac output monitor (NICOM; Cheetah Medical, Tel Aviv, Israel) was used to obtain data regarding advanced hemodynamic parameters during the study period. The NICOM device works on the principle of bioreactance.12 Changes in the intrathoracic blood volume alter the electrical capacitance and inductance properties (bioreactance) of the chest. A 75-kHz current is applied and the relative phase shift of the transthoracic voltage is measured. The cardiac output (CO) is derived from the relative phase shift peak, HR, and ventricular ejection time. The following parameters from NICOM were studied: HR, MAP, SVV, stroke volume index (SVI), CI, and TPRI. The data were collected during the following time-points: before and at the end of study intervention, anesthetic induction (before and 1, 3, and 5 minutes after), tracheal intubation (before and 1, 3, and 5 minutes after), and positioning from supine to prone (before and 5 minutes after). We also collected data regarding baseline CAD using the ANSiscope (Dyansys Inc., Burlingame, CA, United States). The ANSiscope provides ANSindex (degree of dysautonomia), which is derived noninvasively from a recording of 572 RR intervals on an electrocardiogram.13
Conduct of Anesthesia
In the operating room, standard multiparameter monitors were attached. Anesthesia was induced with fentanyl 2 µg/kg, thiopentone 3 to 5 mg/kg, and preservative-free lignocaine 1 mg/kg, and vecuronium 0.15 mg/kg was used to facilitate intubation. Intubation was performed at one minimum alveolar concentration of sevoflurane administered in 50% oxygen-air mixture and anesthesia was maintained at the same level for the study period.
Hypotension during the study period was defined as a MAp < 60 mm Hg or a decrease in MAP by > 20% from the baseline. Hypotension was treated with mephentermine 3 mg boluses, repeated every 5 minutes until the MAP returned to the normal range. The incidence of hypotension, number of episodes of hypotension, total dose of mephentermine used, and total volume of intravenous fluids given during the study period were recorded.
Outcome Measures
Our outcome measures were changes in the HR, MAP, SVV, CI, and TPRI at predefined time points with study intervention (fluid loading), anesthetic induction, tracheal intubation, and prone positioning.
Sample Size Estimation and Statistical Analysis
The sample size was calculated for our primary objective (postinduction hypotension) based on a similar study where the incidence of hypotension was 52% versus 84% at 3 minutes postinduction in preloading versus no preloading groups.14 Considering an α error of 5 and 80% power, 30 patients in each group were deemed appropriate.
The data were analyzed using R software version 3.5.2. Data summaries are depicted as mean and standard deviation (SD) for interval scale variables, median and interquartile range (IQR) for ordinal variables, and frequencies and percentages for nominal variables. Odds ratio (OR) and 95% confidence interval (95% CI) are reported where applicable. Between-group differences were analyzed using Mann–Whitney U test for interval scale and ordinal data, while chi-square test was used for nominal data. Influence of group on trend of change of study variables within predefined time sets was analyzed using linear mixed-effects modeling. Unstructured covariance structure was assumed. For parsimony in analysis, only the first and last time points of each time set were used for modeling. We considered p < 0.05 as level for statistical significance.
Results
All the 60 patients, 30 in each group, completed the study, and the data were analyzed and are depicted in the CONSORT flow diagram (Fig. 1). The demographic parameters and baseline values of ANSindex, hemodynamic, and cardiac dynamic indices are shown in Table 1. There was no difference in demographic variables between the two groups. There was no significant difference between the groups for baseline hemodynamic and cardiac indices except for MAP and CI values. The median volume of intravenous fluids administered in the preloading group was significantly higher as compared with the control group: 1,450 (1,150–1,750) versus 150 (150–187.5), respectively (p < 0.001). The complete descriptive data for various outcome variables in both the groups have been summarized in Supplementary Table S1 (available in online version only).
|
Variables |
Control group (n = 30) |
Preload group (n = 30) |
p-Value |
|---|---|---|---|
|
Age (y) |
45.7 ± 16.3 |
42.4 ± 15.9 |
0.355 |
|
Male gender |
24 (80) |
28 (93.3) |
0.255 |
|
BMI (kg/m2) |
22.6 ± 4 |
22.9 ± 3.4 |
0.450 |
|
Baseline ANSindex |
11.3 ± 19.79 |
13.9 ± 19.59 |
0.616 |
|
Baseline MAP |
97.5 ± 10.7 |
91.6 ± 8.3 |
0.033 |
|
Baseline HR |
75.1 ± 9.2 |
70.4 ± 10 |
0.060 |
|
Baseline CI |
2.2 ± 0.6 |
1.9 ± 0.4 |
0.016 |
|
Baseline SVI |
29.7 ± 6.2 |
27.2 ± 4.1 |
0.149 |
|
Baseline TPRI |
1357.1 ± 535.1 |
1474 ± 496.3 |
0.326 |
|
Baseline SVV |
15.7 ± 3.8 |
16.4 ± 3.1 |
0.536 |
Abbreviations: BMI, body mass index; CI, cardiac index; HR, heart rate; MAP, mean arterial pressure; SVI, stroke volume index; SVV, stroke volume variation; TPRI, total peripheral resistance index.
Note: Data expressed as mean ± SD or median (IQR) or number (percentage).
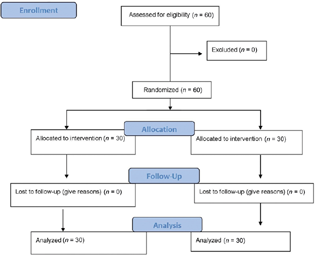
-
Fig. 1 CONSORT flow diagram depicting the flow of the patients into the trial.
Fig. 1 CONSORT flow diagram depicting the flow of the patients into the trial.
The incidence of postinduction hypotension was 26.7% (8/30) and 86.7% (26/30) in patients with and without fluid preloading (p < 0.001). The median (IQR) number of episodes of hypotension during the early intraoperative period was significantly higher in the control group compared with preload group: 3 (1–3) versus 0 (0–0.75), p < 0.001. Fluid preloading decreased the odds of developing hypotension by 71.8% (OR = 0.28; 95% CI, 0.17–0.44; p < 0.001).
The median (IQR) doses (mg) of mephentermine used to treat hypotension were 6 (3–9) and 0 (0–2.25) in group C and group F, respectively (p < 0.001). A linear regression model analysis showed that without preloading, a patient receives a mean dose of 6.1 mg of mephentermine, and the dose reduces by 4.3 mg (95% CI, −6.46 to −2.14) to 1.8 mg with fluid preloading.
The influence of group allocation on the trend of change within time point sets was tested by including interaction of time × group within the regression models. The model estimates and hypothesis tests are provided in the lower panel of corresponding Figs. 1 to 4.
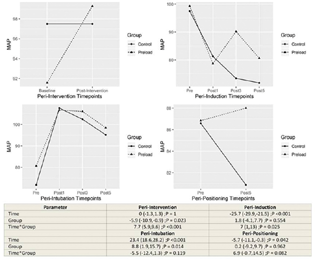
-
Fig. 2 Changes in the mean arterial pressure between control and preload group at different time points.
Fig. 2 Changes in the mean arterial pressure between control and preload group at different time points.
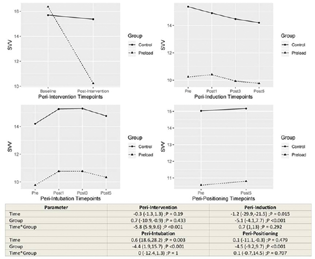
-
Fig. 3 Changes in the stroke volume variation between control and preload group at different time points.
Fig. 3 Changes in the stroke volume variation between control and preload group at different time points.
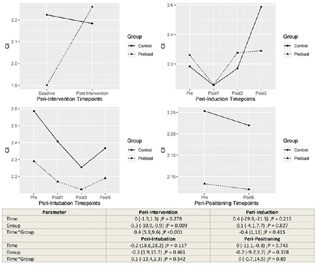
-
Fig. 4 Changes in the cardiac index between control and preload group at different time points.
Fig. 4 Changes in the cardiac index between control and preload group at different time points.
Group F registered an increase in MAP after fluid preloading intervention (p < 0.001). After anesthetic induction, reduction in MAP at 5 minutes was significantly lesser in group F (p = 0.025). Since interaction effects were found to be statistically significant within these time sets, the main effect of group is not discussed. After intubation, group F registered a higher MAP at 5 minutes (main effect p = 0.014). There was no difference in MAP between the two groups after positioning (Fig. 2).
CI was found to increase immediately after the study intervention in the preload group (p < 0.001). Other time sets did not register statistically significant main effect or interaction effect of group on CI (Fig. 3).
TPRI was found to decrease after the intervention in the preload group (p < 0.001). There was no influence of group allocation on the trend of change in TPRI during other time periods (Fig. 4).
The preload group had a significant decrease in SVV after the fluid preloading intervention (interaction effect p < 0.001). The low SVV was maintained through anesthetic induction, tracheal intubation, and prone positioning, with the main effect of group being statistically significant in all the time periods (p < 0.001) (Fig. 5).
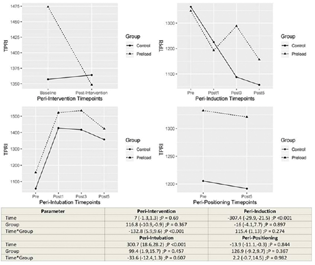
-
Fig. 5 Changes in the total peripheral resistance index between control and preload group at different time points.
Fig. 5 Changes in the total peripheral resistance index between control and preload group at different time points.
Discussion
Preloading and Hypotension in the Early Intraoperative Period
Hypotension after the induction of GA is a common occurrence. If severe and sustained, hypotension during the intraoperative period can lead to adverse consequences. Previous studies have documented benefits of fluid preloading in reducing the incidence of postinduction hypotension. A lower incidence (52 vs. 84%) of postinduction hypotension was observed with 20 mL/kg of Ringer's lactate preloading as compared with no preloading.14 Another study also documented lower incidence (12.5 vs. 57.5%) of postinduction hypotension with 6 mL/kg crystalloid loading as compared with control.15 Similarly, lower incidence (41.5 vs. 56.6%) of postinduction hemodynamic instability was seen with preinduction preloading of 8 mL/kg Ringer's acetate as compared with no preloading.7 In our study, too, we observed a significant difference in the occurrence of hypotension (26.7 vs. 86.7%) with and without fluid preloading. An earlier study demonstrated preoperative volume loading with 8 mL/kg Ringer's lactate to be more effective than preinduction administration of ephedrine in maintaining hemodynamic stability during rapid-sequence induction with propofol without HR increase during intubation.16 Our findings are in concurrence with the observations in previous studies. A comparatively higher incidence of hypotension is likely due to the presence of early CAD in our study population with CCCM. In contrast, a recent RCT comparing SV optimization by fluid loading versus standard fluid therapy before induction of anesthesia did not observe a significant difference in hemodynamic instability in the early intraoperative period.8
Cervical Myelopathy, Autonomic Dysfunction, and Hypotension
The cardiac autonomic system plays an important role in the occurrence of hemodynamic changes during anesthesia.17 The incidence of hypotension and vasopressor use after anesthetic induction for ophthalmic surgery was higher in diabetic patients with CAD compared with nondiabetics without CAD.18 The incidence of postinduction hypotension was 67 to 83% versus 9 to 17% in patients with and without CAD aged ≥40 years undergoing day-care surgery.19 An earlier study documented subclinical CAD in patients with CCCM.20 Patients in both the groups in our study had early CAD. These patients can have an exaggerated hemodynamic response to events such as induction of anesthesia, laryngoscopy and intubation, and prone positioning for surgery. The vulnerability for hypotension during the early intraoperative period increases further in patients with old age, male gender, higher ASA grade, lower MAP, emergency surgery, use of concomitant regional anesthesia, and larger dose of anesthetic induction agent.1 6 There was no difference between the groups for the above-mentioned risk factors in our study and hence the observed differences in postinduction hypotension are likely to be from the presence or absence of fluid preloading.
Effect of Prone Positioning on Hemodynamic and Cardiac Variables
Patients with CCCM develop hypotension during positioning from supine to prone for decompressive spine surgery due to decrease in TPRI.21 In contrast, decreases in SV and CI were responsible for decrease in BP after change from supine to prone position during lumbar spine surgery.22 In an observational study involving 20 patients, preloading with 20 mL/kg Ringer's lactate before positioning from supine to sitting prevented the occurrence of hypotension without significant changes in CO, SVR, SVV, CI, and SV.23 Though the decrease in MAP with prone positioning was more pronounced in patients without preloading in our study, this difference was not statistically significant. Similarly, there was no significant difference in cardiac indices between the groups with change to prone position.
Advanced Cardiac Indices and Anesthesia
An increase in plasma concentration of propofol within the therapeutic range resulted in decrease in MAP, central venous pressure, mean systemic filling pressure, venous return pressure, and SVR, increase in SVV, and no change in CO in 17 patients undergoing upper abdominal surgery.24 Thus, monitoring of CO and associated hemodynamic indices provides better insight into the probable mechanisms for hypotension after GA.
The SVV (%) at baseline was high (16.4 vs. 15.7) in both the groups in our study probably due to low intravascular volume from preoperative fasting. The SVV decreased significantly after fluid loading in the intervention group, while it did not change much in the control group. The SVV remained significantly lower at all the time points of the study period in preload group as compared with no preload group. The SVV decreased after anesthetic induction and increased after intubation and prone positioning in both the groups.
CI decreased from baseline after induction of anesthesia and after prone positioning in patients without preloading, though these changes were not significant. In patients who received preload, CI was maintained above the baseline values at all study time points. However, the difference between the groups was not statistically significant. The effect on CI reflected the combined effect of SVI and HR.
TPRI in both the groups decreased following induction of anesthesia, increased after intubation, and decreased with prone positioning. Preloading did not have any significant effect on the TPRI.
Strengths and Limitations of the Study
To the best of our knowledge, this is the first RCT comparing the effect of fluid preloading on the incidence of hypotension after the induction of GA along with dynamic cardiac indices during the early intraoperative period in patients with early CAD from CCCM. However, an important limitation of this study is restriction of study duration to the early intraoperative period. While the effect of fluid loading on hypotension and other cardiac indices is possible throughout the intraoperative period, other factors such as surgical nociception and blood loss are more likely to affect these outcomes than preanesthetic fluid loading. Second, we defined postinduction hypotension as MAP < 60 mm Hg or a decrease in MAP by >20% from the baseline for this study. The incidences are likely to be different with alternative definitions. Third, the volume of clear fluid intake during the preoperative period was not taken into account and therefore its contribution to postinduction hypotension in both the groups remains unknown. However, in the absence of difference in SVV (a measure of intravascular volume status) at baseline between the groups, influence of preoperative fluid intake is less likely. Fourth, the findings of our study are restricted to thiopentone anesthetic induction with sevoflurane maintenance. Lastly, being a single-center study involving patients with early CAD limits the generalizability of our findings to other populations.
Conclusions
Preloading with 20 mL/kg of Ringer's lactate significantly reduced the incidence of postinduction hypotension in patients with early CAD from CCCM undergoing decompressive surgery. Preloading resulted in an improvement in CI, reduction in SVV and lesser vasopressor use. Use of dynamic cardiac indices in patients with early CAD will help in identifying cause for and managing hemodynamic changes during anesthesia and surgery.
Conflict of Interest
None declared.
Funding Departmental.
References
- Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119(1):57-64.
- [Google Scholar]
- Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107(2):213-220.
- [Google Scholar]
- Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706-721.
- [Google Scholar]
- The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73(10):1223-1228.
- [Google Scholar]
- Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101(3):622-628.
- [Google Scholar]
- Pre-operative fluid bolus for improved haemodynamic stability during minor surgery: A prospectively randomized clinical trial. Acta Anaesthesiol Scand. 2018;62(9):1215-1222.
- [Google Scholar]
- The impact of fluid optimisation before induction of anaesthesia on hypotension after induction. Anaesthesia. 2020;75(5):634-641.
- [Google Scholar]
- Mechanisms contributing to hypotension after anesthetic induction with sufentanil, propofol, and rocuronium: a prospective observational study. J Clin Monit Comput 2021 (e-pub ahead of print).
- [CrossRef] [Google Scholar]
- Pre-anesthetic stroke volume variation can predict cardiac output decrease and hypotension during induction of general anesthesia. J Clin Monit Comput. 2018;32(3):415-422.
- [Google Scholar]
- Bispectral index-guided induction of general anaesthesia in patients undergoing major abdominal surgery using propofol or etomidate: a double-blind, randomized, clinical trial. Br J Anaesth. 2013;110(3):388-396.
- [Google Scholar]
- Effect of intra-arterial nimodipine on cerebral oxygen saturation and systemic hemodynamic indices in patients with cerebral vasospasm: a prospective cohort study. J Neurosurg Anesthesiol. 2020;32(2):177-181.
- [Google Scholar]
- Effect of atropine premedication on cardiac autonomic function during electroconvulsive therapy: a randomized crossover study. J ECT. 2017;33(3):176-180.
- [Google Scholar]
- Prevention of hypotension during induction of anesthesia with propofol and fentanyl: comparison of preloading with crystalloid and intravenous ephedrine. IOSR J Dent Med Sci. 2012;1(1):26-30.
- [Google Scholar]
- Effect of preoperative fluid therapy on hemodynamic stability during anesthesia induction, a randomized study. Acta Anaesthesiol Scand. 2019;63(9):1129-1136.
- [Google Scholar]
- Prophylaxis against the systemic hypotension induced by propofol during rapid-sequence intubation. Can J Anaesth. 1995;42(10):875-878.
- [Google Scholar]
- Pre-operative autonomic nervous system function - a missing link for post-induction hypotension? Anaesthesia. 2022;77(2):139-142.
- [Google Scholar]
- Increased intraoperative cardiovascular morbidity in diabetics with autonomic neuropathy. Anesthesiology. 1989;70(4):591-597.
- [Google Scholar]
- Autonomic reflex dysfunction in patients presenting for elective surgery is associated with hypotension after anesthesia induction. Anesthesiology. 1994;80(2):326-337.
- [Google Scholar]
- Subclinical autonomic nervous system dysfunction in compressive cervical myelopathy. Spine. 2011;36(8):654-659.
- [Google Scholar]
- Haemodynamic changes during prone positioning in anaesthetised chronic cervical myelopathy patients. Indian J Anaesth. 2019;63(3):212-217.
- [Google Scholar]
- Hemodynamic changes during spinal surgery in the prone position. Acta Anaesthesiol Taiwan. 2008;46(2):57-60.
- [Google Scholar]
- Effects of crystalloid preloading (20 ml/kg) on hemodynamics in relation to postural changes in patients undergoing neurosurgical procedures in sitting position. J Neurosci Rural Pract. 2018;9(1):80-85.
- [Google Scholar]
- The effect of propofol on haemodynamics: cardiac output, venous return, mean systemic filling pressure, and vascular resistances. Br J Anaesth. 2016;116(6):784-789.
- [Google Scholar]







