Translate this page into:
Analysis of dynamic changes in optic nerve sheath diameter (ONSD) with ultrasound in post-craniotomy patients: Trends and correlation with computed tomography ONSD and Glasgow coma scale in post-operative period
*Corresponding author: Allan Benhur,Department of Anaesthesiology and Critical Care, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India.benhurallan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Benhur A, Sharma J, Karna ST, Shrivastava A, Saigal S, Waindeskar VV. Analysis of dynamic changes in optic nerve sheath diameter (ONSD) with ultrasound in post-craniotomy patients: Trends and correlation with computed tomography ONSD and Glasgow Coma Scale in post-operative period. J Neurosci Rural Pract 2022;13:676-83.
Abstract
Objectives:
Intracranial pressure (ICP) monitoring in patients with intracranial tumors undergoing craniotomy is usually done in perioperative period in intensive care unit. Invasive measurement of ICP, though considered as the gold standard, has its own limitations such as availability of expertise, equipment, and associated complications. Period of raised ICP in post-operative period may impact patient outcomes. Post-craniotomy computed tomography (CT) assessment is done routinely and may need to be repeated if indicated during post-operative stay. Utility of sonographic serial optic nerve sheath diameter (ONSD) assessment in post-operative monitoring of patients who have undergone elective craniotomy was explored in this study. The primary objective of the study was to measure the dynamic change in ONSD as compared to baseline pre-operative measurement in the first 3 postoperative days after elective craniotomy. The secondary objective of the study was to evaluate correlation between ONSD value with Glasgow Coma Scale (GCS) and post-operative CT findings.
Materials and Methods:
In this prospective, observational, and cohort study, we studied adult patients undergoing craniotomy for intracranial tumors. GCS assessment and sonographic measurement of ONSD were done preoperatively, immediate post-operative period, and 12, 24, and 48 h after surgery. CT scan to detect raised ICP was done at 24 h post-operative. Correlation of ONSD with GCS at respective period and correlation of CT scan finding with respective ONSD assessment were evaluated.
Results:
A total of 57 patients underwent elective craniotomy for intracranial tumors. Significant difference was observed in ONSD value depending on time of measurement perioperatively (χ2 = 78.9, P = 0.00). There was initial increase in the first 12 h followed by decrease in ONSD in the next 48 h. Negative correlation was observed between baseline ONSD and 12 h GCS (ρ = −0.345, P = 0.013). There was significant change in GCS scores based on the status of ONSD (raised or normal) at 12 h after surgery (P = 0.014). Significant correlation between USG ONSD and CT ONSD was observed (ρ = 0.928, P = 0.000). Optimal cutoff value of ONSD to detect raised ICP with reference to CT signs was 4.8 mm with 80% sensitivity and 95% specificity.
Conclusion:
ONSD undergoes dynamic changes, correlates with CT scan, and has good diagnostic accuracy to detect raised ICP post-craniotomy for intracranial tumors. It may serve as a useful tool in monitoring in resource-limited setup.
Keywords
Optic nerve sheath diameter
Intracranial pressure
Glasgow coma scale
Craniotomy
Intracranial tumor
Computed tomography scan
INTRODUCTION
Patients with intracranial tumors undergoing intracranial surgery have risk of post-operative intracranial pressure (ICP) elevation and associated clinical deterioration.[1,2] This can lead to significant morbidity and mortality if not timely diagnosed and intervened. Pre-operative monitoring of neurological status may be done by the assessment of Glasgow Coma Scale (GCS) or radiological methods such as computed tomography scan (CT scan) or magnetic resonance imaging (MRI). However, each of these methods is associated with shortcomings after surgery, especially in a mechanically ventilated patient. Although GCS has been in use since 1974, it lacks reliability in intubated patients. Radiological assessment of cerebral edema perioperatively by CT scan or MRI is cumbersome with risk of untoward events during transfer to the radiological suites that may lead to significant morbidity and mortality. Further, even invasive measurement of ICP needs specialized equipment and expertizes along with risk of exposure to infection.[3-5]
The optic nerve sheath is the continuation of the dura mater and subarachnoid space that contain cerebrospinal fluid (CSF). Intracranial cavity is in direct continuation with the CSF filled subarachnoid space between optic nerve and optic nerve sheath. Hence, an increase in CSF pressure due to brain edema would expand the sheath. Optic nerve sheath diameter (ONSD) does not significantly alter with age, gender, or geographic origin.[6] It is possible to assess ONSD by CT and MRI, but the issue of mobilizing a post-operative patient is a major drawback.
Sonographic assessment of ONSD has been verified in various intensive care-based clinical studies as a non-invasive monitor for changes in ICP.[7-9] It is simple, safe, inexpensive, and repeatable, bedside technique for the detection of raised ICP. However, there is a paucity of clinical studies exploring the utility of serial measurements of sonographic ONSD in the post-operative period, correlation with CT findings of ONSD, and influence on neurological status in terms of GCS in patients undergoing elective craniotomy for intracranial tumor.
Thus, we conducted this study with the primary objective of analysis of dynamic changes in the ONSD measured by sonography in the perioperative period in patients undergoing elective craniotomy. The secondary objective was – (a) to study the influence of raised ONSD on GCS score and (b) to evaluate the correlation of sonographic measurement of ONSD with that measured by CT scan at 24 h after surgery.
MATERIALS AND METHODS
Study design and subjects
This prospective, single-center, observational, and cohort study was done at a tertiary care teaching institute in Central India after the Institutional Ethical Committee approval (IHEC No-LOP/2018/MD0015). Written informed consent was given by all patients included in the study.
Consecutive hospitalized adult patients scheduled for elective craniotomy under general anesthesia above 18 years and below 70 years of age over a period of 15 months from November 1, 2018, to January 31, 2020, were recruited. A total of 57 patients were recruited. Patients with any disease precluding assessment of ONSD by ultrasound, such as pregnancy with pre-eclampsia, hypertension (severe), diabetes mellitus (severe), glaucoma, known optic nerve disease, pituitary tumors, ASA Grade IV or V, ophthalmic diseases, such as inflammations, tumors, or trauma, were excluded from the study.
Data collection
After informed consent by each patient, a dedicated study investigator assessed the GCS and the ONSD by ultrasonography (USG) at predefined time points in the perioperative period – immediately before and after completion of surgery as well as 12, 24, and 48 h after surgery.
Sonographic ONSD measurements were done by single-trained observer to eliminate interobserver bias, using the M-Turbo ultrasound image system with a 6–13 MHz linear array probe (Sonosite Inc., Bothell, WA, USA). The probe was gently placed on the superior and lateral aspect of the orbit against the upper eyelid with the eye closed and angled slightly caudally and medially until the optic nerve was visualized as a linear hypoechoic structure with clearly defined margins posterior to the globe. All images were obtained in a transverse/ axial plane for uniformity. The ONSD was measured 3 mm behind the retina [Figure 1]. At each period of measurement, three readings of each eye were taken and the average of the six readings was considered as the final ONSD measurement, to decrease intraobserver bias. Furthermore, at each period of measurement, GCS score was assessed. Intraoperatively, all patients were monitored with an electrocardiogram, pulse oximeter, and non-invasive blood pressure/invasive blood pressure and temperature monitor (35–37°C). Anesthetic management was as per standard institutional protocol. Anesthetic induction was done with injection thiopentone sodium 5 mg/kg and fentanyl 1.5 mcg/kg using vecuronium 0.6 mg/kg to facilitate tracheal intubation. Propofol infusion was used to maintain anesthetic depth along with supplemental doses of fentanyl. After intubation, lung was ventilated with oxygen and air mixture with fractional inspired oxygen concentration of 40% with 6–8 ml/kg tidal volume and the respiratory rate being adjusted to maintain an end-tidal carbon dioxide partial pressure of 3.5–4.0 kPa during surgery.

- Measurement of optic nerve sheath diameter in ultrasonography.
At end of surgery, extubation trial was given only if the following prerequisites were fulfilled
Duration of craniotomy is <6 h
Intraoperative hemodynamics remained stable
Presence of pulsatile brain
ONSD measurement immediately after craniotomy does not change >25% of baseline.
In case, extubation trial failed or above-mentioned conditions were not met, post-operative invasive mechanical ventilation was electively done in intensive care unit (ICU).
All patients underwent routine post-craniotomy CT on postoperative day 1 as per institutional protocol. The findings of routine post-operative CT were evaluated by neuroradiologist blinded to the sonographic ONSD findings. Neuroradiologist measured ONSD in the CT image, 3 mm behind the globe similar to measurement of USG ONSD [Figure 2] and also assessed for signs of raised ICP in CT. The evaluation for the presence of elevated ICP was based on the following criteria: Midline shift from mass effect of 3 mm or greater, collapse of third ventricle, hydrocephalus, effacement of sulci with evidence of significant edema, optic nerve tortuosity, collapse of mesencephalic cisterns, or evidence of herniation. All CT ONSD measurements and evaluation of signs of raised ICP in CT were assessed by single radiologist.

- Measurement of optic nerve sheath diameter in computed tomography.
Statistical methods
Data analysis was done with IBM SPSS Statistics (v23). The distribution of the collected data was evaluated for normality to ascertain the required statistical tests for analysis using descriptive analysis of SPSS. The results of the analyses showed that the collected data did not follow a normal distribution and tests were applied accordingly. Change in mean ONSD at different time intervals (baseline, immediate post-operative, and at 12, 24, and 48 h) was evaluated using Friedman test (non-parametric alternative for repeated measures ANOVA) and Wilcoxon signed-rank test (non-parametric alternative to the t-test).
Correlations between ONSD and GCS were assessed with Kruskal–Wallis test and Spearman correlation test. Evaluation of correlation between ONSD measurement by sonography and by CT scan was assessed by Spearman correlation test. Agreement between the two methods was assessed by plotting Bland-Altman plot and Cohen’s kappa was calculated. Diagnostic properties of USG ONSD to predict raised ICP were analyzed with CT signs of raised ICP as reference. Receiver operating characteristic (ROC) curve was plotted and Youden’s index (YI= Sensitivity+Specificity–1) was used to determine the optimal cutoff on USG ONSD to predict raised ICP, with reference to CT signs of raised ICP. USG ONSD measured at 24 h post-operative period was used for analyzing diagnostic properties and the reference CT was routine post-operative CT done on post-operative day 1 (24 h post-operative) in which signs of raised ICP were evaluated.
RESULTS
Patient demographics
We recruited a total of 57 patients, of which six patients were excluded from the study, due to transfer to another center (three), refusal to participate (one), and inability to get CT scan done at 24 h due to logistic reasons (two) [Figure 3]. Out of the 51 patients included in data analysis, 31 were males and 20 were females. Majority (65%) belonged to middle age group (20–50 years), with the mean age of 37.5 years. The tumor was supratentorial in 40 patients (28 were intra-axial and 12 were extra-axial) while 11 had infratentorial tumor (five were intra-axial and six were extra-axial). All patients underwent craniotomy and excision or decompression surgery.
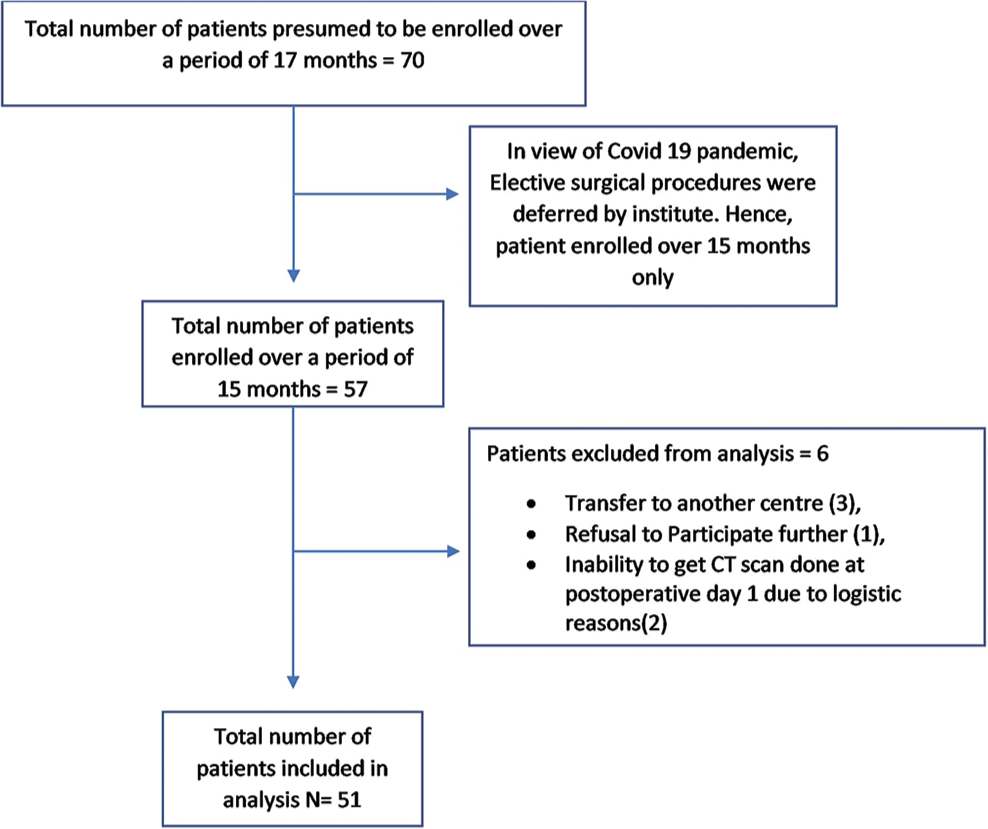
- Study flowchart.
Trend of changes in measured ONSD values
We observed that the ONSD undergoes dynamic changes at different time points studied perioperatively. However, a very statistically significant change in ONSD values was observed between the values taken before and immediately after surgery (P = 0.00). There was also statistically significant change in ONSD between baseline (pre-operative) ONSD and 12 h post-operative ONSD (P = 0.002). No statistically significant change in ONSD values measured was observed between baseline (pre-operative) ONSD and 24 h or 48 h after surgery(P = 0.236 and 0.279, respectively).
Median ONSD values were found to be increased between baseline (pre-operative) ONSD and immediate postoperative ONSD, and between baseline (pre-operative) ONSD and 12 h post-operative ONSD. Whereas, the median ONSD values measured were found to be decreased between immediate post-operative ONSD and 12 h post-operative ONSD, 12 h post-operative ONSD and 24 h post-operative ONSD, 24 h post-operative ONSD and 48 h post-operative ONSD [Figure 4].

- Box and Whisker plot of optic nerve sheath diameter (ONSD) measurement at different time of measurement. Depicting median ONSD and interquartile range at each period of measurement.
ONSD and GCS
ONSD values were grouped into raised and normal using conventional cutoff value of 5 mm. At 12 h post-operative period, there was statistically significant difference (P = 0.014) in GCS scores in raised ONSD group whereas not in the normal ONSD group. This change in distribution of GCS scores between raised and normal ONSD group was not observed at other time points of measurement in the post-operative period.
Significant negative correlation was observed between baseline (pre-operative) ONSD and GCS at 12 h post-operative period (Spearman correlation coefficient = −0.345, P = 0.013).
USG ONSD and CT ONSD
Significant correlation was observed between ONSD measured by sonography and in routine post-operative CT done 24 h after surgery with a correlation coefficient of 0.917, P = 0.000 on the left side and a correlation coefficient of 0.954, P = 0.000 on the right side. The average correlation coefficient of the left and right side was 0.928, P = 0.000.
Furthermore, significant agreement between the two methods, USG ONSD and CT ONSD, was observed on Bland-Altman plot with narrow limits of agreement = ±0.318 (95% intervals 0.358, −0.280, mean difference = 0.038) [Figure 5].

- Ultrasonography optic nerve sheath diameter (ONSD) and computed tomography ONSD – Bland-Altman plot.
Thus, the two methods, that is, sonography and CT scan assessment of ONSD, are essentially equivalent with a very small average discrepancy between methods (bias). Furthermore, Cohen’s K agreement analysis showed good agreement between two modalities of measurements, by K = 0.922, P = 0.000
Diagnostic properties of ONSD for predicting raised ICP
Diagnostic properties of ONSD measurement by USG (ONSD at 24 h post-operative period) to identify raised ICP were analyzed with signs of raised ICP on CT scan done 24 h after surgery as reference. The ROC showed excellent discrimination to detect raised ICP with area under the curve (AUC) of 0.9, (P = 0.000, 95% CI = 0.78–1.000) [Figure 6]. The subsequent optimal cutoff value of ONSD to detect raised ICP was observed to be 4.833 mm, with a sensitivity of 80% and specificity of 95.1%.
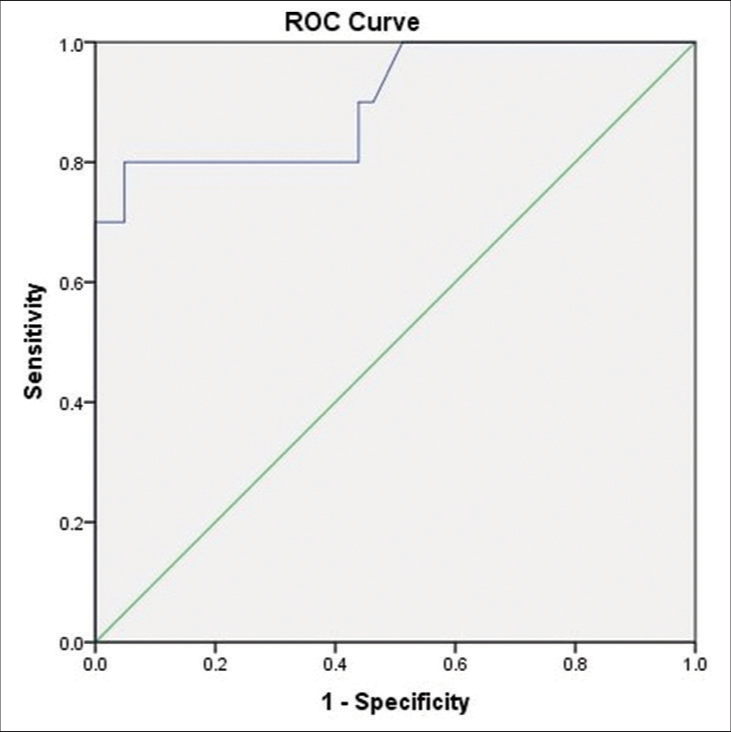
- ROC curve of ultrasonography optic nerve sheath diameter for detecting raised intracranial pressure (ICP) with reference to computed tomography signs of raised ICP.
DISCUSSION
In this prospective, observational, and cohort study done on 51 patients undergoing elective craniotomy for intracranial tumor, we observed that
There is a dynamic change in ONSD assessed sonographically in the post-operative period with a statistically significant difference in ONSD from baseline pre-operative value to immediately after and 12 h after surgery.
At 12 h after surgery, a statistically significant difference (P = 0.014) in neurological status (GCS score) was present when ONSD was >5 mm.
Sonographic assessment of ONSD is equivalent to the value by CT scan with the presence of a very small average discrepancy between methods by Bland-Altman plot and good agreement on Cohen’s K agreement analysis.
The sonographic ONSD has an excellent discrimination for the detection of raised ICP at 24 h after surgery with AUC of 0.9 and cutoff ONSD value of 4.8833 mm, with a sensitivity of 80% and specificity of 95.1%.
Correlation of ONSD with ICP is well established in various studiesand ONSD has been extensively studied in patients requiring close neurological monitoring with risk of raised ICP.[9-18] It has been used for the prediction of raised ICP before neurosurgical proceduresand even during non-neurosurgical procedures (steep Trendelenburg position in robotic-assisted laparoscopic surgeries, other laparoscopic procedures,in overweight parturient with intracranial tumor).[19-23] ONSD has been utilized in evaluation of idiopathic intracranial hypertension, spontaneous intracranial hemorrhage, traumatic brain injuries, acute mountain sickness, cerebral edema, and brain death.[11,14,19,24-33]
There have been contrasting studies on the utility of perioperative serial measurements of ONSD in patients undergoing elective craniotomy for intracranial tumor, with this research area being not well explored. Gao et al. in their prospective and observational study raised concerns regarding value of ONSD in 33 patients after hemicraniectomy.[34] They hypothesized that skull defects may theoretically alter the dynamics of CSF circulation or structure of optic nerve sheath, thus questioning the reliability of post-operative ONSD. However, only one measurement of ONSD was done at 6 h after surgery along with concurrent ICP and correlation was made. Further, even this study observed that raised ONSD on the side opposite to craniectomy correlated with poor outcome after surgery. In the study by Wang et al., ONSD and ICP were measured at 6 and 24 h after surgery in patients who underwent decompressive craniotomy, a strong correlation was observed between ONSD and invasive ICP measurements.[19] In another study by Raffiz et al., in adult traumatic and non-traumatic neurosurgical patients, a significant correlation was found between ONSD and ICP.[28] Post-operative sustained ICP elevation is a potential risk in patients undergoing intracranial surgery for tumor resection;[1,2] hence, ONSD monitoring was done in post-operative period.
The timing and interval of the assessment of ONSD have also been different across studies with measurements done either in pre-operative phase[20] or at 1 time point like 24 h after surgery in post-operative stage.[35] In our study, we assessed the dynamic change in ONSD from baseline pre-operative value till the 3rd post-operative day. Hence, measurements were done at five different instances; at pre-operative (baseline), immediate post-operative, and at 12, 24, and 48 h post-operative period. The median values of ONSD showed increasing trend from baseline pre-operative to immediate post-operative ONSD value (P = 0.00), whereas statistically significant decreasing trend was observed every 12 hourly till 24 h and 24 hourly till 48 h after surgery [Figure 4]. Our findings are similar to that observed by Majhi et al. with significant difference between pre-operative and post-operative ONSD measurement.[35] Among all patients with raised ONSD, we observed highest percentage of raised ONSD during the immediate postoperative period and 12 h after surgery. These data reinforce the need for close monitoring of ICP during the early postoperative period, especially till 12 h after surgery.
Overall sensitivity and specificity of ONSD measurement in predicting raised ICP in neurosurgical patients were higher in traumatic group (sensitivity – 94.4% and specificity – 95.2%) compared to non-traumatic group (sensitivity – 83.3% and specificity – 93.3%) in an observational study by Raffiz et al.28 The sensitivity observed in our study is in agreement with their value in non-traumatic group of patients. Increased sensitivity in low-risk (low prevalence) population and increased specificity in high-risk (high prevalence/high clinical suspicion) population were found in a meta-analysis by Ohle et al.[36] on use of ONSD for the detection of raised ICP compared to CT, in which 12 studies with 478 participants were included in the study. Considering patients in our study as having high prevalence of raised ICP due to possibility of post-operative cerebral edema, the findings of high specificity (95.1%) of ONSD value in our study support their observation.
The cutoff value of sonographic ONSD which indicates raised ICP is a matter of debate. In a prospective and observational study of 43 patients by Mohammed et al.,[32] where ONSD was evaluated as a screening test in comatose ICU patients, a higher value of 5.8 mm was observed to be associated with cerebral edema, with a sensitivity of 86% and specificity of 74%. Moretti et al.[26] studied ONSD to detect raised ICP in patients of spontaneous intracranial hemorrhage. In the 94 ONSD measurements, they observed, a value of 5.2 mm was found to be optimal ONSD cutoff to detect raised ICP with sensitivity of 93.1% and specificity of 73.85%.
However, with direct measurement of ICP by invasive methods, the most common cutoff ONSD value of 5 mm has been correlated with raised ICP.[10,37-40] In an observational study in elective craniotomy patients, Shafiq et al.[20] compared the diagnostic accuracy of pre-operative sonographic ONSD for detecting elevated ICP in comparison with CT scan and clinical signs. They also used 5 mm as the cutoff for raised ONSD and observed an 80% diagnostic accuracy when compared to radiographic evidence of raised ICP on CT scan. Similarly, we also used an ONSD value of 5 mm to classify patients into raised or normal ICP groups. However, the subsequent cutoff value of ONSD for the detection of raised ICP on plotting the ROC curve was observed to be 4.833 mm, with a sensitivity of 80% and specificity of 95.1%.
Usually, post-operative neurological status is evaluated by CT scan in most centers. However, CT scan is limited by risks and cost involved. In a meta-analysis comparing detection of raised ICP by USG ONSD and CT scan by Ohle et al.,[36] the former performed exceptionally well for the identification of raised ICP with a sensitivity of 95.6% and specificity of 92.3%. These findings support credibility of usage of USG ONSD for evaluation of raised ICP, in place of CT ONSD. We observed similar findings with good agreement and correlation between USG ONSD and CT ONSD.
In our institute, most patients are sedated and electively ventilated in the immediate post-operative period, and planned for extubation between 12 and 24 h postoperatively based on the neurological status and improvement in GCS. In our study, the distribution of GCS scores varied significantly (P = 0.014) between raised (>5 mm) and normal (<5 mm) ONSD groups at 12 h post-operative period. Furthermore, statistically significant negative correlation (correlation coefficient = −0.345, P = 0.013) was observed between pre-operative ONSD and GCS at 12 h postoperatively. Hence, USG ONSD status can be used as an additional data to decide on extubation by observing the descending trends of ONSD of patients during this post-operative period.
No statistically significant change in GCS score based on status of ONSD (raised or normal) was observed in any other period of measurements in our study. However, the absence of correlation between GCS and ONSD in our study is attributable to other factors such as use of post-operative elective sedation, muscle relaxation, and mechanical ventilation in ICU in immediate post-operative period, as only 4% of patients were extubated immediately after surgery.
Limitations of the study
Although the gold standard for ICP assessment is invasive measurement by intraventricular catheter systems, invasive ICP measurement was not done, considering well-established correlation between ONSD and ICP, risks involved in invasive ICP measurement and the primary intent of our study being to explore the clinical utility of non-invasive method – ONSD in monitoring for raised ICP and assess correlation with routine CT in post-operative period.
All ONSD measurements were done by single observer. The possibility of intraobserver variability exists. However, multiple measurements were performed with the average used as the final measurement to reduce bias due to intraobserver variability.
USG ONSD correlation with GCS was affected in immediate post-operative period in view of elective sedation and mechanical ventilation. Thus, we could not find statistically significant correlation during this period.
All intracranial tumors were evaluated together without differentiating into supratentorial or infratentorial tumors, as there is no available evidence on effect on ONSD by the location of intracranial tumor; moreover, requirement of in-depth understanding of anatomical location of tumor was beyond the scope of the study. Further studies should incorporate effect of location of tumor on ONSD perioperatively to have a more specific prognostic value.
CONCLUSION
Our prospective and observational study in elective craniotomy for intracranial tumor has shown that there is a dynamic change in ONSD in the perioperative period with significant rise in the first 12 h of post-operative period and followed by a decline in ONSD.
When pre-operative baseline ONSD was higher, postoperative GCS scores at 12 h were lower (negative correlation). Hence, pre-operative ONSD may act as an additional prognostic tool in determining post-operative duration of mechanical ventilation and ICU stay.
Significant difference in GCS scores was observed depending on ONSD status (raised or normal) in post-operative period (at 12 h). Post-operative monitoring of ONSD may serve as an additional factor in guiding decision for tracheal extubation.
The optimal cutoff value of ONSD to predict raised ICP, with reference to CT signs of raised ICP, was determined as 4.833. Having a good correlation between USG ONSD, CT ONSD, and other CT findings of raised ICP, ONSD may be used in routine post-operative monitoring of patients undergoing craniotomy for brain tumor. This may help to assess risk of elevated ICP and may be used instead of invasive ICP monitoring/CT scan in resource-limited settings.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Intracranial pressure monitoring after elective intracranial surgery: A retrospective study of 514 consecutive patients. J Neurosurg. 1988;69:540-4.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial pressure monitoring in postoperative treatment of supratentorial and infratentorial gliomas. In: Richard KE, Radebold K, eds. Chemotherapy of Gliomas. Berlin, New York: Walter de Gruyter; 1985.
- [Google Scholar]
- Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg Focus. 2017;43:E6.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial Pressure and Neuromonitoring in Brain Injury Vienna: Springer; 1998.
- [CrossRef] [Google Scholar]
- Intracranial pressure monitoring: Invasive versus non-invasive methods-a review. Crit Care Res Pract. 2012;2012:950393.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification of optic nerve and sheath diameter by transorbital sonography: A systematic review and metanalysis. J Neuroimaging. 2020;30:165-74.
- [CrossRef] [PubMed] [Google Scholar]
- Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506-15.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015;123:743-7.
- [CrossRef] [PubMed] [Google Scholar]
- The correlation between endocranial pressure and optic nerve diameter: An ultrasonographic study In: Documenta Ophthalmologica Proceedings Series. Netherlands: Springer; 1987. p. :603-6.
- [CrossRef] [Google Scholar]
- Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15:201-4.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2011;37:1059-68.
- [CrossRef] [PubMed] [Google Scholar]
- Use of the sonographic diameter of optic nerve sheath to estimate intracranial pressure. Am J Emerg Med. 2013;31:236-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective analysis of single operator sonographic optic nerve sheath diameter measurement for diagnosis of elevated intracranial pressure. West J Emerg Med. 2014;15:217-20.
- [CrossRef] [PubMed] [Google Scholar]
- Transocular ultrasound measurement of the optic nerve sheath diameter can identify elevated intracranial pressure in trauma patients. Trauma. 2016;18:28-34.
- [CrossRef] [Google Scholar]
- Assessment of intracranial pressure with ultrasonographic retrobulbar optic nerve sheath diameter measurement. BMC Neurol. 2017;17:188.
- [CrossRef] [PubMed] [Google Scholar]
- Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2018;44:1284-94.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between ultrasonographic optic nerve sheath diameter and intracranial pressure. Zhonghua Yan Ke Za Zhi. 2018;54:683-7.
- [Google Scholar]
- Ultrasonographic optic nerve sheath diameter to detect increased intracranial pressure in adults: A meta-analysis. Acta Radiol. 2019;60:221-9.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonographic optic nerve sheath diameter correlation with ICP and accuracy as a tool for noninvasive surrogate ICP measurement in patients with decompressive craniotomy. J Neurosurg. 2019;133:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pre-anaesthetic assessment of intracranial pressure using optic nerve sheath diameter in patients scheduled for elective tumour craniotomy. J Ayub Med Coll Abbottabad. 2018;30:151-4.
- [Google Scholar]
- Changes in intraocular pressure and optic nerve sheath diameter in patients undergoing robotic-assisted laparoscopic prostatectomy in steep 45 Trendelenburg position. BMC Anesthesiol. 2017;17:40.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of pneumoperitoneum and Trendelenburg position on intracranial pressure assessed using different non-invasive methods. Br J Anaesth. 2016;117:783-91.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonographic optic nerve sheath diameter measurement in overweight parturient with intracranial tumour: Guiding choice of anaesthesia. Indian J Anaesth. 2016;60:775-7.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic assessment of the optic nerve sheath diameter in the diagnosis of idiopathic intracranial hypertension. J Neurol Sci. 2016;361:122-7.
- [CrossRef] [PubMed] [Google Scholar]
- Optic nerve sonographic examination to predict raised intracranial pressure in idiopathic intracranial hypertension: The cut-off points. Neuroradiol J. 2018;31:490-5.
- [CrossRef] [PubMed] [Google Scholar]
- Reliability of optic nerve ultrasound for the evaluation of patients with spontaneous intracranial hemorrhage. Neurocrit Care. 2009;11:406-10.
- [CrossRef] [PubMed] [Google Scholar]
- Transorbital ultrasonographic measurement of optic nerve sheath diameter for intracranial Midline shift in patients with head trauma. World Neurosurg. 2016;85:292-7.
- [CrossRef] [PubMed] [Google Scholar]
- Optic nerve sheath diameter measurement: A means of detecting raised ICP in adult traumatic and non-traumatic neurosurgical patients. Am J Emerg Med. 2017;35:150-3.
- [CrossRef] [PubMed] [Google Scholar]
- Role of serial ultrasonic optic nerve sheath diameter monitoring in head injury. Neurochirurgie. 2017;63:444-8.
- [CrossRef] [PubMed] [Google Scholar]
- Optic nerve sheath diameter, intracranial pressure and acute mountain sickness on Mount Everest: A longitudinal cohort study. Br J Sports Med. 2008;42:183-8.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound measurement of ocular nerve sheath diameter can be used for the estimation of cerebral oedema. Balkan Mil Med Rev. 2014;17:45.
- [Google Scholar]
- Optic nerve sheath diameter by bedside ultrasound is a reliable screening test for cerebral edema in the comatose ICU patient. Crit Care. 2015;19:P457.
- [CrossRef] [Google Scholar]
- Transorbital ultrasonographic measurement of optic nerve sheath diameter in brain death: Transorbital ultrasonographic measurement of ONSD in BD. J Neuroimaging. 2015;25:906-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and prognostic value of the optic nerve sheath diameter with respect to the intracranial pressure and neurological outcome of patients following hemicraniectomy. BMC Neurol. 2018;18:199.
- [CrossRef] [PubMed] [Google Scholar]
- Transorbital ultrasound measurement of optic nerve sheath diameter. A novel technique for measuring intra-cranial pressure in neurosurgical intensive care setup. Indian J Appl Res. 2020;10:1.
- [Google Scholar]
- Sonography of the optic nerve sheath diameter for detection of raised intracranial pressure compared to computed tomography: A systematic review and meta-analysis. J Ultrasound Med. 2015;34:1285-94.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003;10:376-81.
- [CrossRef] [PubMed] [Google Scholar]
- Can ocular ultrasound predict intracranial hypertension? A pilot diagnostic accuracy evaluation in a UK emergency department. Eur J Emerg Med. 2013;20:91-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound measurement of optic nerve sheath diameter in patients with a clinical suspicion of raised intracranial pressure. Emerg Med J. 2011;28:679-81.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of optic nerve ultrasonography in head injury. Injury. 2008;39:519-24.
- [CrossRef] [PubMed] [Google Scholar]







