Translate this page into:
Cystic hemangioblastoma of the brainstem
Address for correspondence: Dr. Amit Agrawal, Department of Neurosurgery, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe), Wardha- 442 005, Maharashtra, India. E-mail: dramitagrawal@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hemangioblastomas are very highly vascular neoplasm with benign characteristics and; in comparison to cerebellar hemangioblastoma; cases of cystic hemangioblastoma of the brain stem are rare with only a few case reports available in the literature. We report the case of a 43-year-old-female with cystic hemagioblastoma of the brainstem managed successfully and review the relevant literature.
Keywords
Cystic hemangioblastoma
fourth ventricle
hemangioblastoma
medulla oblongata
Introduction
Hemangioblastomas are very highly vascular neoplasm with benign characteristics representing between 2-3% of brain tumors and; only 5-20% of hemangioblastomas are located in the brainstem.[1–4] The junction of the medulla and upper spinal cord is a less common site where these lesions are diagnosed.[134] In comparison to cerebellar haemangioblastoma, cystic hemangioblastoma of the brain stem is rare with only a few case reports in literature (the size of mural nodules varies between 6 and 20 mm in diameter).[135–8]
Case Report
A 43-year-old-female presented with a history of progressive weakness, difficulty in swallowing and staggering gait of six months duration. Her general and systemic examination was normal except for bilateral papilloedema. Neurologically, she was conscious, alert and oriented to time, place and person. Cranial nerves examination was normal. There were no focal motor or sensory deficits. There was no neck rigidity or other signs of meningeal irritation. Her routine blood investigations were normal. Magnetic resonance imaging (MRI) showed a mass lesion that was hypo intense on T1W images and hyper intense on T2W images, occupying the caudal part of the fourth ventricle and the dorsal medulla, associated with a cystic component and obstructive hydrocephalus. The caudal medulla was displaced ventrally and the vermis was displaced dorsally and superiorly [Figures 1–2]. While waiting for surgery, she deteriorated in sensorium and became progressively drowsy. She was undertaken for emergency suboccipital craniectomy and removal of the mass lesion.
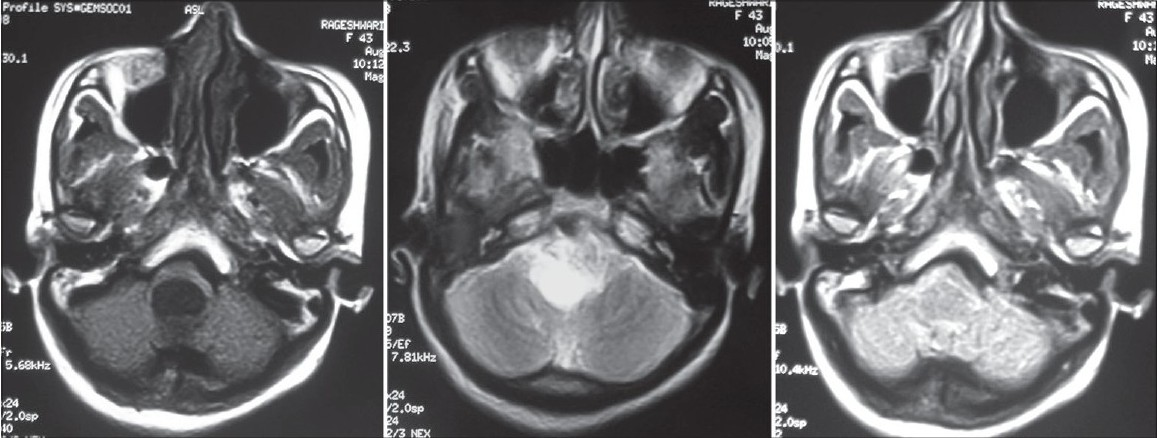
- MRI brain axial T1W, T2W and FLAIR images showing well defi ned cystic lesion at the level of medulla oblongata
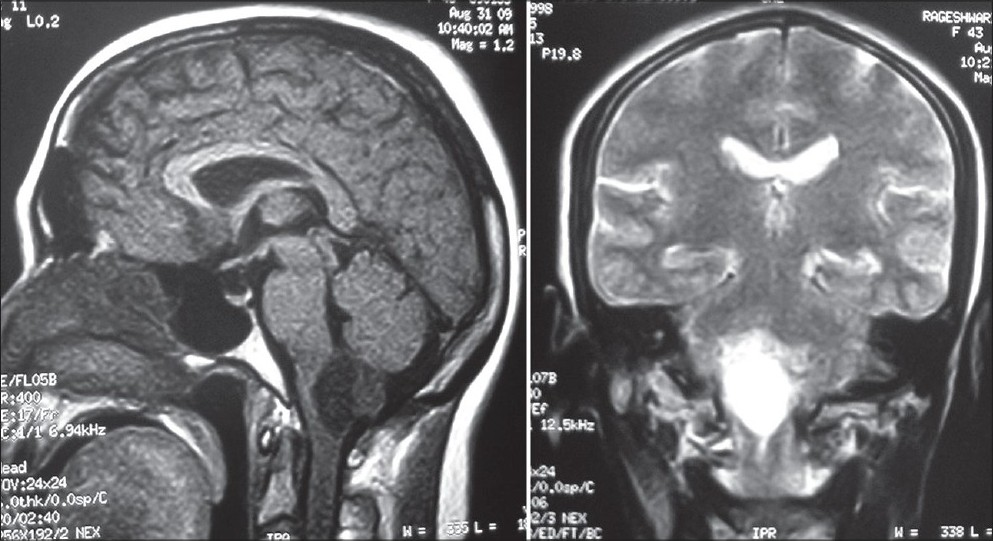
- MRI brain T1W axial and T2W coronal images showing well defi ned cystic lesion at the level of medulla oblongata with a dorsally located mural nodule
After opening the dura there was cherry red, highly vascular mass lesion was found on the dorsal surface of the medulla oblongata, obstructing the foramen of Magendie with dilated abnormal veins [Figure 3]. Under the operating microscope, a plane of cleavage, formed by gliotic interspace between the tumor and the brain was identified, the ventral feeding arteries were identified, coagulated with bipolar forceps, and divided and the tumor could be completely dissected from the brain stem. Postoperatively, her vital signs were stable; she regained full consciousness without respiratory distress or dysarthria or any focal neurological deficits and could be easily extubated. Histopathology confirmed the diagnosis of hemangioblastoma [Figure 4]. At follow-up the patient is doing well.
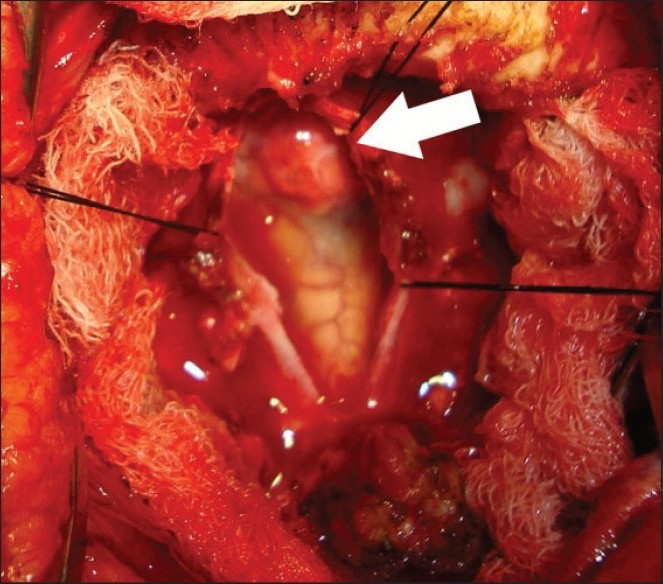
- Intra-operative photograph showing a cherry red lesion arising from the lower medulla oblongata
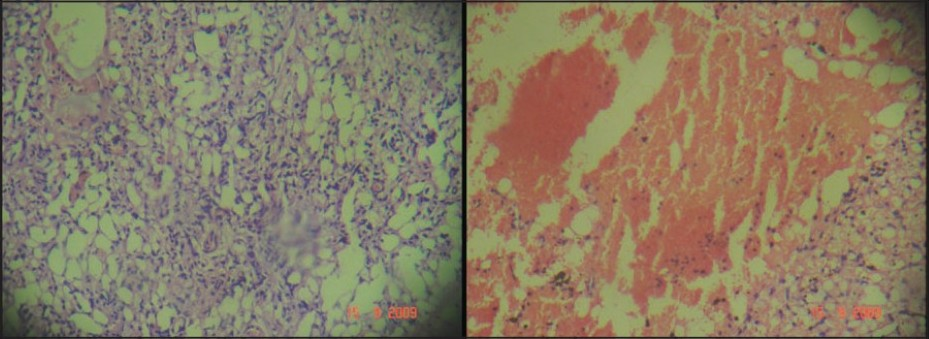
- Photomicrograph showing features of hemangioblastoma and hemorrhage (H & E, ×40)
Discussion
It has been postulated that the hemangioblastoma of the fourth ventricle originates from a vascular mesenchymal plate in or adjacent to the posterior end of the floor of the ventricle (posterior medullary velum), which develops in the third month of fetal life.[59] According to their location, hemangioblastomas of the brain stem can be categorized into three locations: Type A (hemangioblastoma of the fourth ventricle attached to the floor of the ventricle), Type E (hemangioblastoma of the fourth ventricle partially embedded in the floor of the ventricle, common one), and Type I (intramedullary hemangioblastoma of the medulla oblongata).[10] Identification of these lesions according to two locations, fourth ventricle and intramedullary, is helpful in assessing the operative risk and functional quality of the clinical results clinical outcome,[4610] however, whether surgical procedures and the results vary significantly with site remains unclear.[6]
MRI is the investigation of choice and is usually sufficient for preoperative evaluation.[211] Cerebral angiography is recommended as it will allow a detailed study of the vascular anatomy and will help while planning of a surgical strategy,[2] however presurgical embolization is unnecessary.[11] The goal of surgery is complete resection of the lesion before the patient develops disabling neurological deficits.[11] With the incorporation of the latest neuroimaging and microsurgery techniques, complete and safe microsurgical resection of brain stem hemangioblastomas, without any kind of post-operative neurological deficits is feasible.[1241011] However, postoperative care is mandatory to reduce the operative mortality and morbidity (due to the likelihood of respiratory and circulatory disorders, and bulbar palsy).[1012–14] The solid nodule tumor mass is removed microscopically by dissecting the gliotic margins away from the tiny vascular pedicles that are coagulated, however the removal of the cyst wall is not necessary.[15] A cleavage plane is often present between the tumor and the floor of the fourth ventricle and this cleavage separates the tumor from the brain parenchyma and facilitating tumor removal.[101617] It has been recommended that to prevent catastrophic bleeding, piecemeal removal must be avoided and to avoid intraoperative swelling, the large draining veins have to be preserved until the arterial feeders to the mural nodule have been isolated and resected.[15]
Source of Support: Nil
Conflict of Interest: None declared.
References
- Cystic hemangioblastoma of the junction of the medulla and upper spinal cord associated to von Hippel-Lindau disease: Two case reports and a review of the literature. Rev Neurol. 2009;48:463-8.
- [Google Scholar]
- Hemangioblastomas of the spinal cord and the brainstem: diagnostic and therapeutic features. Neurosurg Rev. 1996;19:147-51.
- [Google Scholar]
- Diagnosis and surgical treatment of brainstem hemangioblastomas. Surg Neurol. 2005;63:307-15.
- [Google Scholar]
- A case of cystic hemangioblastoma of the medulla oblongata. No Shinkei Geka. 1996;24:949-53.
- [Google Scholar]
- Radiographic diagnostic evaluation and surgical treatment of multiple cerebellar, brain stem, and spinal cord hemangioblastomas. Surg Neurol. 1978;9:337-41.
- [Google Scholar]
- Haemangioblastoma, haemangioblastomatosis, and von Hippel-Lindau diseases. In: Symon L, Calliauw L, Cohadon F, Antunes JL, Loew F, Nornes H, eds. Advances and Technical Standards in Neurosurgery. Vol 20. New York: Wien, Springer; 1993. p. :197-304.
- [Google Scholar]
- Intramedullary hemangioblastoma of the medulla oblongata--two case reports and review of the literature. Neurol Med Chir (Tokyo). 1998;38:489-98.
- [Google Scholar]
- Surgical management of brainstem hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:95-105.
- [Google Scholar]
- Surgery for gliomas and other mass lesions of the brain stem. In: Symon L, Calliauw L, Cohadon F, Dolenc VV, Antumes IL, Nornes H, eds. Advances and Technical Standards in Neurosurgery. Vol 22. New York: Wien, Springer; 1994. p. :261-341.
- [Google Scholar]
- Successful total removal of intramedullary hemangioblastoma from the medulla oblongata. Surg Neurol. 1993;39:25-30.
- [Google Scholar]
- Hemangioblastoma of the cervicomedullary junction.Report of three cases. J Neurosurg. 1986;64:317-21.
- [Google Scholar]
- Intracranial hemangioblastomas: An institutional experience. Neurol India. 2006;54:276-8.
- [Google Scholar]
- Surgical removal of hemangioblastoma in the fourth ventricle. Surg Neurol. 1980;13:423-7.
- [Google Scholar]






