Translate this page into:
Cranioplasty: Review of materials and techniques
Address for correspondence: Dr. Bashar Abuzayed, Department of Neurosurgery, Cerrahpasa Medical Faculty, Istanbul University, Cerrahpasa Street, 34089, Fatih, Istanbul, Turkey. E-mail: sylvius@live.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cranioplasty is the surgical intervention to repair cranial defects. The aim of cranioplasty is not only a cosmetic issue; also, the repair of cranial defects gives relief to psychological drawbacks and increases the social performances. Many different types of materials were used throughout the history of cranioplasty. With the evolving biomedical technology, new materials are available to be used by the surgeons. Although many different materials and techniques had been described, there is still no consensus about the best material, and ongoing researches on both biologic and nonbiologic substitutions continue aiming to develop the ideal reconstruction materials. In this article, the principle materials and techniques of cranioplasty are reviewed.
Keywords
Allograft
autograft
cranioplasty
reconstruction
skull defects
Introduction
Cranioplasty is the surgical intervention to repair cranial defects in both cosmetic and functional ways. The history of cranioplasty dates back to 7000 B.C.[1] Archeologic findings proved that the use of inorganic materials for cranioplasty had begun before the organic materials.[1] In 19th century, the use of bone from different donor sites, such as ribs or tibia, gained wide population. Although many different methods had been described, there is still no consensus on which method is better. In this article, the principle materials and techniques of cranioplasty are reviewed.
Indications and Timing in Cranioplasty
Cranioplasty is performed mostly after traumatic injuries. With children younger than 3 years old, growing skull fractures and congenital anomalies are common causes. In all age groups, tumor removal or decompressive craniectomies are mostly the cause of cranial defects. The aim of cranioplasty is not only a cosmetic issue; also, the repair of cranial defects gives relief to psychological drawbacks and increases the social performances. Moreover, the incidence of epilepsy is shown to be decreased after cranioplasty.[2] On the other hand, contraindications for cranioplasty are the presence of hydrocephalus, infection, and brain swelling. In children below 4 years old, if there is an intact dura mater, cranium can achieve self closure. Waiting to perform cranioplasty is important to prevent the development of devitalized autograft or allograft infections. It is generally accepted to wait 3 to 6 months before reconstructive surgery. If there is an infected area, this waiting period can be as long as one year.
Materials and Techniques in Cranioplasty
Many different types of materials were used throughout the history of cranioplasty. With the evolving new biomedical technology, new materials are now available to be used by the surgeons.
An ideal cranioplasty material must have the following features:[3]
-
It must fit the cranial defect and achieve complete closure
-
Radiolucency
-
Resistance to infections
-
Not dilated with heat
-
Strong to biomechanical processes
-
Easy to shape
-
Not expensive
-
Ready to use
-
Still, there is no perfect material to fit all these criteria.
Autografts in Cranioplasty
Cranium
Macewen (1885) and Burrell (1888) used the remaining calvarial bone after trepanation.[4] In 1890, Muller developed the “sliding flaps” technique of the external tabula, which was applied in the late postoperative period.[4] The first example of bone transplantation is the technique of Söhr, in which he used only the external tabula of cranium without periosteum.[45] Although the use of external tabula is a considerable way of cranioplasty, the use of internal tabula is rather new.[6] Split-thickness skull cranioplasty are biocompatible, which are easy harvested and with less infection and reaction risks. For this reason, it is considered a good option for cases with high risk of infection.[7] In pediatric patients whom skull growth is continuing, split-thickness skull grafts showed integration and cooperated with the remolding skull, in contrast to fixed nonbiologic materials which resulted in restricted growth of the skull and deformities in adult ages.[8]
Tibia
The first cranial reconstruction in an aesthetic aspect was performed by lying tibia pieces between periosteum and dura mater.[5] The first patient series belongs to Exhausen, who treated 27 patients with this method.[5] Recently, the use of tibia is seldom, because harvesting is difficult and traumatic for patient. Also, cranial contour cannot be obtained easily with tibia graft.
Rib
This method was popularized at the beginning of the 20th century. However, many surgeons do not prefer using ribs, because of the intra- and postoperative complications of the technique, such as deformities of thorax and respiratory problems.[59]
Scapula
Although scapula is a good option as an autologous bone graft, it is no more used. This is due to the difficulty and high complication rate from harvesting this graft.
Fascia
With soft tissues such as temporal muscle or fascia, only small areas of bone defects can be closed. On the other hand, their usefulness in duraplasty cannot be despised. Dural repair with vascularized dural grafts and flaps was preferred by many neurosurgeons because of their healing ability and effective defect closure.[10] Muscle and omental grafts are considered to be rich vascularized grafts, and applications for the reconstruction of the skull base surgery and cerebral revascularization have been reported. Pedicled local flaps including pericranial and galeal flaps are not thick enough to prevent erosion and cannot be used in a patient who had undergone previous multiple craniotomies as in complicated cases.[10] Non-pedicled fascia provides less protection against infection and is not suitable after repeated craniotomies in which the risk of meningitis is high. Many factors determine the success of duraplasty, such as the normal or low intracranial pressure, the viabilities of the graft and the dura, and the young age of the patient [Figure 1]. Intracranial pressure elevation can be prevented by cerebrospinal fluid (CSF) divergence with a lumbar catheter placed postoperatively, which is a common practice in neurosurgery. In addition, it is important to provide viable tissue for duraplasty, which will result in healthy healing of the graft and/or flap with consequent closure of the defect and prevention of the CSF leakage.[10] This may be sometimes difficult in certain situations, such as repeated operations on one site, previous cranial radiotherapy, chemotherapy, and systemic diseases that may interfere with normal wound healing, such as anemia, low-cardiac output diseases, hypoproteinemia and hypoalbuminemia, hypovitaminosis, smoking, and diabetes.[10] In these situations, it is recommended to use the most viable tissue available to perform duraplasty that may overcome these problems. Flaps are superior to grafts in defect repair because of their patent blood flow from the pedicle and resultant healthy healing. The best graft known to be suitable for duraplasty are autologous fascia and muscle grafts. Compared with synthetic grafts, autologous grafts are more viable and with less tissue reaction.[10]

- Intraoperative view of one of our cases demonstrating the application fascia lata graft. (B: boney skull, F: fascia lata)
Sternum
Sternum is a mixed cortical cancellous graft. This graft is not widely used due to its disadvantages, such as the lack of sufficient volume to cover the cranial defects and difficult and complicated harvesting. Also, it is more porous in nature, more rapidly revascularized, and therefore more rapidly resorbed.[11]
Ilium
Ilium was a preferred autologous bone graft because of similarity to the contour of the cranium. However, due to complications, such as hemorrhage, bowel perforation, and nerve damage, the use of ilium for cranioplasty became unpopular. Also, the mixed cortical cancellous iliac graft is more porous in nature, more rapidly revascularized, and therefore more rapidly resorbed.[11]
Protection of autografts
Many techniques were suggested for the protection of autografts when it is improper to replace the bone flap after craniotomy. The main considerations of these techniques are to use patients’ own bone tissue to achieve bony closure and to keep bone flap “alive” in waiting period. Westerman proposed to use craniotomy materials after boiling in water.[1] But after high infection rates, this method was abandoned. Another method is autoclaving to prevent infections. However, it was seen that the bone could not keep its viability after autoclaving in most cases.[1] The most recent method to protect autografts is to freeze the bones. Dry freeze in -70°C is an accepted way to keep bone flaps sterile and ready to use. This technique keeps the matrix architecture of bone intact and ready to use. But this technique does not prevent the bone from “dying.” Saving the craniotomy flap in the fatty tissue of the abdomen was first described by Kreider in 1920.[1] This method is no more as popular as it was first described, because the need for a second surgery arises, the scar tissue in abdomen occurs, and osteogenic capacity of the bone is never as it is expected. However, saving the graft in abdominal fat is still preferred by many surgeons and is the most preferred method in our institution.
Allograft
First use of allografts was by Morestin in 1915 with cartilage of cadaveric origin.[12] Cadaveric cartilages were popularized during the World War I due to their elastic nature and high resistance to infections. But with time, their use decreased because it did not show calcification as expected and did not provide enough mechanical protection.[12] Also, cadaveric cranial bones have been used for the purpose of cranioplasty. But, this method was not popularized, because of high infection rates. Even after proper sterilization, the use of cadaveric cranial bones can end up with reactions to foreign substance.
Xenograft
Historically, animal bones have been widely used to close cranial defects. Meereken used a dog's cranial bones to achieve closure of cranial defects in 1682.[13] Interestingly, in 1917, scapulae of cows obtained from hospital meals have been used in cranioplasty named as “soup bone.” Although there are some good results with xenografts, they are no more widely used.
Non-metal Allografts
Celluloids
Celluloids were widely used until the discovery of tantalum and methyl-methacrylate. The main disadvantage was postoperative fluid collection and the need of aspiration of this fluid.[3]
Methyl-methacrylate
After World War II, there was a large need for cranioplasty. Acrylic was primarily a substance used by dentists. Therefore, it was evolved into the use in cranioplasty.[14]
Acrylic has some advantages above metal substances; it is easy to shape, lighter in weight, radiates less heat, and radiolucent. Acrylic in the form of methyl-methacrylate (polymethylmethacrylate) was first used in animal models, and then in human beings in the first years of the World War II. Animal experiments revealed that acrylic adheres to the dura mater with no reaction to other underlying layers.[15] Methyl-methacrylate was widely used after the article of Spence in 1954.[3] With time, aiming to prevent undesired breakings of this material, it was tried to give structural support with steel or titanium meshes. Before the use of methyl-methacrylate, scalp adhered to dura is removed gently and clean bone borders are achieved. Methyl-methacrylate is then prepared in a proper form with curvature. After installment, methyl-methacrylate must be washed with cool water to prevent heat damage to the adjacent brain tissue. After this step, methyl-methacrylate is placed in a cup filled with physiologic serum to finish cooling and hardening. The material is fixed to the bone with miniplates. When it is attempted to use methyl-methacrylate with titanium mesh, titanium mesh must be fixed with miniplates first, then it is poured in liquid form. Again, proper cooling is achieved with water. Methyl-methacrylate is the most extensively used cranioplasty material.[1516]
Hydroxyapatite
Hydroxyapatite is made up of hexagonal form of calcium phosphate.[17] This material is already present in bone tissue; thus, it is believed that hydroxyapatite increases bone repair.[17] The advantages of hydroxyapatite are minimal tissue reaction, increased bone repair, and good osteointegration. On the other hand, the most prominent disadvantage is that this material is not very resistant to mechanical stress and can easily break.[1617] Recently, porous structure gave this material more osteointegrative state and its use with titanium mesh made hydroxyapatite more durable. It is suggested that patients with hydroxyapatite cranioplasty should stay away from trauma until total bone repair.[17]
Polyethylene and silicon
Silicon was proposed as a cranioplasty material in 1968, but its soft build limited its use [Figure 2].[18] Polyethylene is a material used in insulation of electric cables in planes. In the middle of 20th century, it was started to be used as a cranioplasty material. Especially its easy shapeable build with heat made this material popular.[81920] Porous polyethylene sheet has an excellent biocompatibility, reflected by the rarity of known allergic reactions and by the favorable response of tissues to its surfaces.[81920] The open-pore characteristic allows porous polyethylene's early vascularization, followed by soft tissue ingrowth and collagen deposition [Figure 3].[81920] These features offer superior advantages against infection. Consistent with most other alloplastic implants, if infection does occur, treatment is possible with systemic antibiotics rather than by the removal of the implant.[81920]
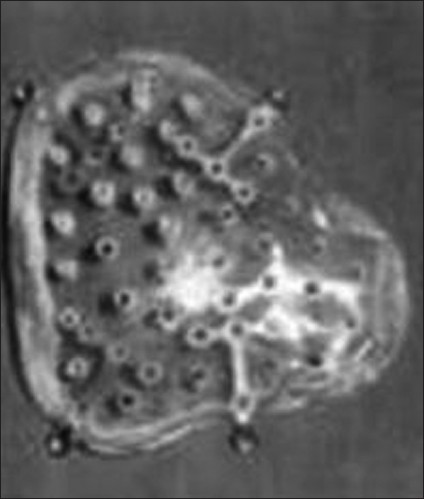
- Silicon cranioplasty kit
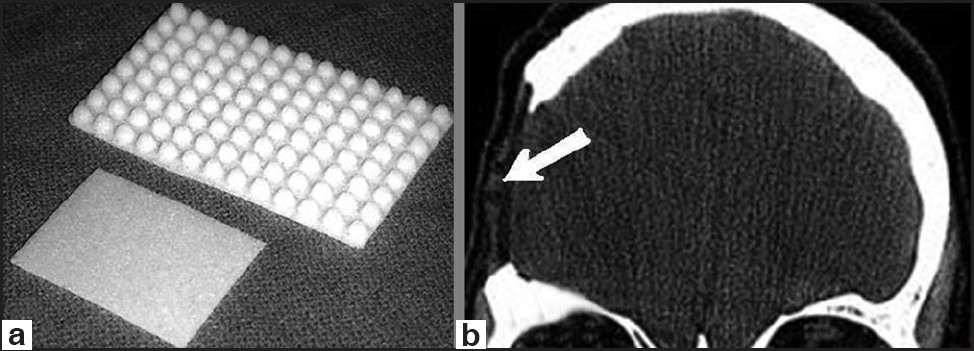
- (a) Porous polyethylene cranioplasty kit types, (b) postoperative head CT-scan of one of our cases demonstrating the defect reconstructed with porous polyethylene (arrow)
Chorale
This material can be found in the skeleton of many sea creatures. Like hydroxyapatite, it increase bone repair and generates great bone fusion.[21] Its main disadvantage is its insufficient durability.
Ceramic
Ceramic as a cranioplasty material is rather new.[16] Its osteointegration is similar to acrylic, however; its main disadvantage is its insufficient durability.
Cortoss
Cortoss™ (Orthovita®, Malvern, USA) is a new synthetic bone void filler that contains bis-glycidyl-methyl-methacrylate, bisphenol (a polyethylene glycol diether dimethylacrylate), triethylene glycol dimethylacrylate monomer, and bioactive glass ceramic.[22] It is provided in a double lumen cartridge with specially designed tips for mixing. After the composite is expressed through these tips, polymerization begins and the material is ready for use. The monomer is not volatile and Cortoss™ polymerizes in a three-dimensional network, which minimizes the chances of leaking.[22] After mixing, the material has the consistency of toothpaste, and stays that way until it polymerizes in a matter of seconds or minutes. During polymerization, mixing Cortoss™ with blood prolongs the hardening time, which leads to easy application [Figure 4].[22] This characteristic provides a consistent tactile feedback and allows for an even injection. Cortoss™ has shown that it caused less exothermic reaction and maximum polymerization was at 40°C, closest to the biological conditions (37°C).[22] The modulus of elasticity of Cortoss™ is close to that of bone. This composite is bioactive, and the cement-bone interface continues to be strengthened over time with bone apposition occurring at the interface without any fibrous interposition.[22] Periosteal and endosteal bones were seen at Cortoss™ repaired sites. New bone was seen in areas where blood vessels had grown directly adjacent to Cortoss™ but no vascular invasion was seen.[22] Cortoss™ causes lower rate of inflammation. Cortoss™ has been shown to exhibited higher values for compressive strength, bending modulus, and shear strength.[22]
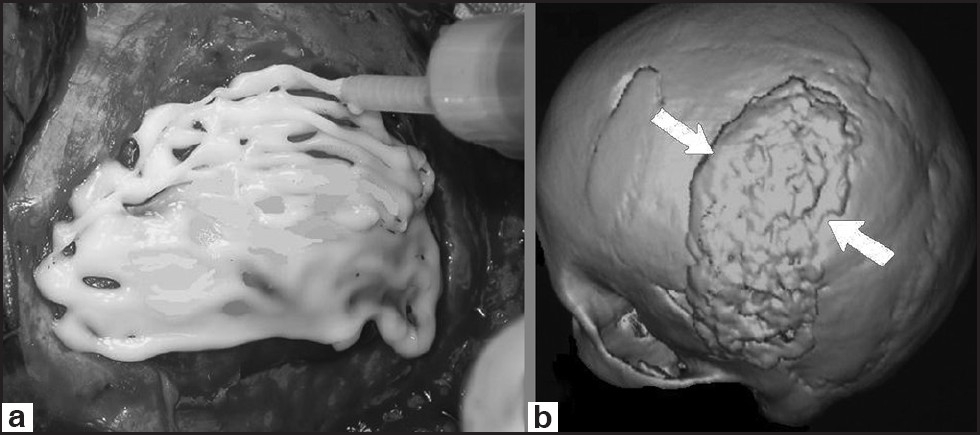
- One of our cases with skull defect reconstructed with Cortoss™. (a) Intraoperative view of Cortoss™ use, (b) postoperative head 3D CT-scan demonstrating the defect reconstructed with Cortoss™ (arrows)
Metal allografts
Their heat conduction, difficulty to shape, and radio-opacity limited their use as a proper cranioplasty material.
Aluminum, gold, and silver
Historically, the Incas used gold and silver in cranioplasty.[1] On the other hand, in modern ages, aluminum was the first metal used in cranioplasty.[17] Aluminum showed infectious complications in many occasions. Also, many patients suffered from epilepsy after cranioplasty with aluminum. With time, the use of Aluminum for cranioplasty vanished due to these complications.[7]
Although gold gave good results as a cranioplasty material with low complication rates and ease to shape, the main problem with gold is that it is a very expensive metal. Silver was first used by Sebileau in 1903.[1] However, the use of silver was left due to its disadvantages. Silver materials are soft and could not provide mechanical protection. Also, oxidization of silver caused color changes in the overlying skin.[1] Silver was tried to be used as mesh plates, but this also resulted in unfavorable results.[1]
Tantalum
Tantalum was widely used in World War II, but abandoned due to its high price, difficulty to obtain, and the main complication of headache, probably because of high heat conduction ability.[23]
Stainless steel and titanium
The flexible structure of steel and deformities in the material seen after minor traumas prevented its use in large defects. Titanium is hard to shape, but relatively cheaper, bioacceptable, and radiolucent after mixing with other metals [Figure 5].[24] It also showed good resistance to infection, even when in contact with the paranasal sinuses.[24] However, it is not a good option in cases with bad skin viability (eg: multiple operations, radiotherapy, etc.).[22] Recently, titanium meshes were used as a support to cement materials. In this way, the strong resistance against mechanical stress of the titanium and the ability to remodeling of the cement materials were combined.
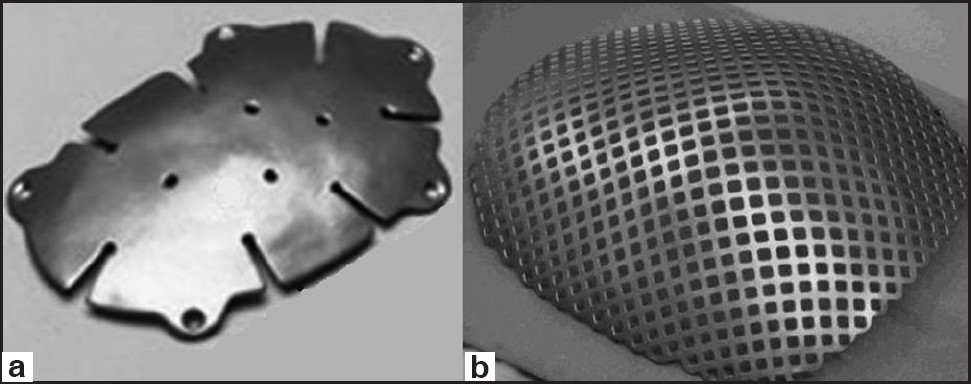
- Titanium cranioplasty kit. (a) Titanium plate, (b) titanium mesh
Lead and platinum
Lead was used for the first time as a cranioplasty material at the beginning of 20th century. However, it leaded to toxicity and related deaths. For this reason, the use of lead was banded. Platinum showed good biocompatibility with no tissue reaction. However, its use was not widespread due to its expensive coasts.[1]
Vitallium and ticonium
Vitallium composes cobalt, molybdenum, and chrome. It was already used as a dental implant and showed minimal corrosion. After experiments in animals which showed that compound metals give less tissue reaction than pure metals, vitallium was popularized in cranioplasty.[1] Ticonium is similar to vitallium but it contains also nickel. Its ease to give shape and lightness are advantages over vitallium.[1316]
Endoscopic cranioplasty
The development in endoscopic equipment and techniques gives the surgeons the opportunity of a minimal invasive cranioplasty. With endoscopic tools, materials such as acrylic, hydroxyapatite, and choral can be administrated through small incisions. Although minimal invasiveness is an advantage, there is still lack of large patient groups to support this method.
Nuances in cranioplasty
Most cranioplasty materials are used with little adjustments by the surgeon. But the basic principle must not be forgotten; to choose proper material for the defect. A cranioplasty material must have low infection rates, show low heat conduction, to be non-magnetic, radiolucent, tissue acceptable, durable, shapeable, and inexpensive.[3] Before achieving bone closure, clear bone borders should be obtained, and scalp should be dissected from dura. Dural tears should be closed in watertight manner. Bone and cranioplasty material should touch to each other with maximum capacity. To prevent the mobility of the cranioplasty flap, the material is fixed to the bone with proper plates.
Future in cranioplasty
Perfection in cranioplasty is still not achieved, and ongoing researches on both biologic and nonbiologic substitutions continue with the help of recent technology. Stem cell experiments and development of morphogenic proteins are expected to take place in the short-term future.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Repairing holes in the head: A history of cranioplasty. Neurosurgery. 1997;40:588-603.
- [Google Scholar]
- Cranioplasty: A review of 1030 cases of penetrating head injury. Neurosurgery. 1979;4:381-5.
- [Google Scholar]
- Autogenous skull cranioplasty: Fresh and preserved (frozen), with consideration of the cellular response. Neurosurgery. 1979;4:18-29.
- [Google Scholar]
- Reconstruction of the supraorbital ridge using aluminum. Surg Neurol. 1978;9:121-8.
- [Google Scholar]
- Reconstruction of growing skull fracture with in situ galeal graft duraplasty and porous polyethylene sheet. J Craniofac Surg. 2009;20:1245-9.
- [Google Scholar]
- Successful use of rib grafts for cranioplasty in children. Pediatr Neurosurg. 2001;34:149-55.
- [Google Scholar]
- Duraplasty using autologous fascia lata reenforced by on-site pedicled muscle flap: Technical note. J Craniofac Surg. 2009;20:435-8.
- [Google Scholar]
- Volume maintenance of inlay bone grafts in the craniofacial skeleton. Plast Reconstr Surg. 2003;112:802-11.
- [Google Scholar]
- The use of bone grafts and alloplastic materials in cranioplasty. Clin Orthop Relat Res. 1991;268:270-8.
- [Google Scholar]
- The prognosis of cranioplasty following large craniectomy in children. Z Kinderchir. 1988;43:375-83.
- [Google Scholar]
- [Substitution of skull defects with methyl acrylate] Magy Traumatol Orthop Helyreallito Seb. 1976;19:259-68.
- [Google Scholar]
- Mechanical characterization of bone graft substitute ceramic cements. Injury 2011 Mar 1
- [Google Scholar]
- Hydroxyapatite cement paste cranioplasty for the treatment of temporal hollowing after cranial vault remodeling in a growing child. J Craniofac Surg. 1997;8:506-11.
- [Google Scholar]
- Cranioplasty with a silicone prosthesis and split rib grafts. Head Neck Surg. 1986;8:355-62.
- [Google Scholar]
- Aneurysmal frontal bone cyst in a child with history of acute lymphoblastic leukemia: A case of rare location and history. J Pediatr Hematol Oncol. 2010;32:e1-3.
- [Google Scholar]
- Intraosseous meningioma: A rare tumor reconstructed with porous polyethylene. J Craniofac Surg. 2010;21:936-9.
- [Google Scholar]
- Osteogenesis with coral is increased by BMP and BMC in a rat cranioplasty. Biomaterials. 1999;20:1909-18.
- [Google Scholar]
- Use of Cortoss as an alternative material in calvarial defects: The first clinical results in cranioplasty. J Craniofac Surg. 2008;19:88-95.
- [Google Scholar]
- Tantalum cranioplasty of war wounds of the skull. Ann Chir Gynaecol Fenn. 1949;38:13-9.
- [Google Scholar]
- Long-term results following reconstruction of craniofacial defects with titanium micro-mesh systems. J Craniomaxillofac Surg. 2001;29:75-81.
- [Google Scholar]






