Translate this page into:
Role of Thalamus in Recovery of Traumatic Brain Injury
Address for correspondence: Dr. Ashok Munivenkatappa, National Institute of Epidemiology, Chennai, Tamil Nadu, India. E-mail: ashokmphdns@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Degree of recovery after traumatic brain injury is highly variable that lasts for many weeks to months. The evidence of brain structures involved in recovery mechanisms is limited. This review highlights evidence of the brain structure particularly thalamus in neuroplasticity mechanism. Thalamus with its complex global networking has potential role in refining the cortical and other brain structures. Thalamic nuclei activation both naturally or by neurorehabilitation in injured brain can enhance and facilitate the improvement of posttraumatic symptoms. This review provides evidence from literature that thalamus plays a key role in recovery mechanism after injury. The study also emphasize that thalamus should be specifically targeted in neurorehabilitation following brain injury.
Keywords
Anisotropy
neuroplasticity
posttraumatic symptoms
recovery
thalamus
traumatic brain injury
INTRODUCTION
Neuroplasticity following traumatic brain injury
Traumatic brain injury (TBI) is a critical public and medical health problem, which causes morbidity and mortality, especially among productive age group. Although TBI is commonly used all over, no two brain injuries are same in any aspect. TBI is heterogeneous in manifestation that may be due to mechanism of injury, force by which patient's brain was hit, environmental factors, and patient factors (age, genetic, etc). Posttraumatic symptoms can be compared to spectrum of symptoms that affect daily activities of life. As such, the degree of recovery is highly variable, with some individuals regaining complete or near complete functions while others are disabled. There is uncertainty in recovery mechanism following brain injury.[1234] In any severity of brain injury, there is substantial spontaneous recovery that occurs over weeks to months after injury. The human brain is capable of widespread structural plasticity leading to restoration of function. There is experimentation that has demonstrated many anatomical and physiological examples of neuroplasticity.[3456] During this neuroplasticity phase, few brain parts play a potential role in enhancing and facilitating recovery process.[3] Among them, thalamus, a subcortical brain structure with its global network connections plays a key role.[78]
METHODS AND DISCUSSION
Thalamic connections and its functions in neuroplasticity following traumatic brain injury
The thalamus is a deep-seated subcortical structure, that is, situated in the forebrain above the midbrain. The thalamus nuclei play an important role in coordinating and maintaining consciousness in human beings.[91011] A positron emission tomographic study on vegetative state, TBI patients showed that there was a significant difference in modulation between thalamic nuclei, prefrontal, and anterior cingulate cortices as compared with healthy controls. After recovery, the thalamocortical modulation was same as controls. The study supports that thalamocortical connections are associated in the maintenance of consciousness.[9] Thalamic nuclei receive ascending fibers from brain stem/basal forebrain, that is, arousal system that regulates cortical activities. The same thalamic neurons are innervated from descending fibers from cortices. Collectively, the nuclei of thalamus appear to modulate the level of arousal and alertness that is important to carry out cognitive task and maintain wakeful state.[111213]
The thalamus with its complex global network connections has anatomical and physiological specialization that supports its key role in consciousness,[9] arousal,[11] cognition,[14] behavior,[15] working memory,[16] executive function,[11] motor control,[17] sustained, and vigilant attention.[18] The recent microcircuit proposes that central thalamic neurons inhibit GABAergic pallidal neurons which in turn have tonic inhibition on central thalamic neurons, therefore, creating positive feedback loop. The thalamocortical and thalamostriatal show progressive deafferentation in proportion to severity of structural brain injuries, causing disinhibition of GABAergic pallidal neurons which in turn cause persistent tonic inhibition of central thalamic nuclei as a whole the thalamic network connection decreases.[101920] The concept of central thalamus deep brain stimulation (CT-DBS) is to activate thalamostriatal and thalamocortical network connections to regulate and facilitate thalamic function.
Thalamic nuclei with its ascending and descending fibers regulate and facilitate many physiological functions. Few studies have evidenced that thalamus by natural means,[7821] rehabilitation means,[222324] and by invasive stimulation method[5] have crucial role in recovery mechanism. However, the exact evidence and mechanism for thalamic global dynamic network are not understood in detail.
Preclinical and histopathological studies evidence of thalamus with its network connection in recovery of traumatic brain injuries
In rodents, electrical stimulation of central thalamus is linked with activation of cortical, striatal, hippocampal neural circuits. This network activation is associated with improvements in learning, working memory, arousal system, and waking of rat from asleep.[102526] Recovery of somatosensory function among rats was associated with intact myelinated fibers connecting thalamic nucleus and somatosensory cortex as was evidenced by blood-oxygen-level-dependent response of magnetic resonance imaging (MRI) scan.[27] TBI rats treated with allopregnanolone had shown better performance in spatial performance task; allopregnanolone, a metabolite of progesterone, has shown to enhance gamma-aminobutyric acid neurotransmission in mediodorsal nucleus of thalamus.[28]
Electrophysiological studies of alert monkeys have shown that central thalamus is directly involved in regulating firing pattern for performing a task and increases in firing pattern while performing task.[29] In a histopathological study, one patient with diffuse axonal injuries (DAI) and transneuronal degeneration with no structural damage to thalamus had survived for more months as compared to other DAI patients.[30] Reason for the same is not known.
Imaging evidence: Thalamic role in recovery of cognitive domains, postconcussion symptoms, pain, and behavioral functions following brain injuries
There are very few studies that have provided evidence of brain structures and connections that are involved in recovery following brain injuries [Table 1].
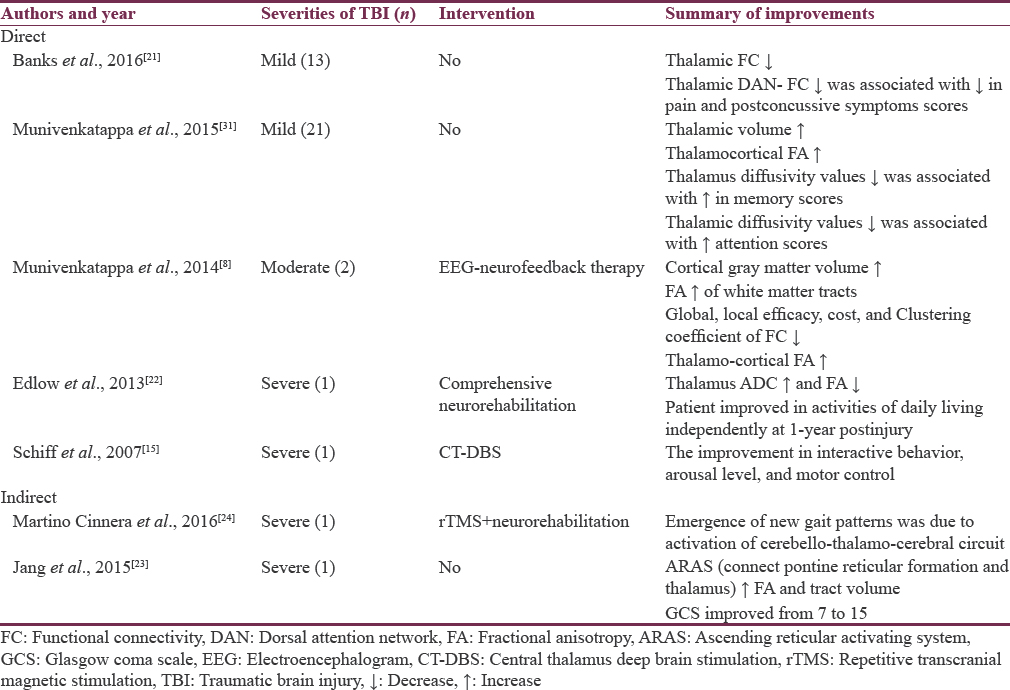
Direct evidence
Thirteen mild TBI patients at 4 months showed that thalamic functional connectivity (FC) was normalizing to baseline as compared to impaired 6 weeks thalamic FC. The decrease in patient's pain severity and postconcussion symptoms was significantly associated with normalization of thalamic dorsal attention network FC at 4 months.[21] A three time-point imaging study on mild TBI patients hypothesized that increase in mean thalamic volume from baseline and appearance of fractional anisotropy of thalamocortical connection after 3–4 months of injury was significantly associated with improvements in cognitive domains.[31] Two moderate TBI pediatric patients after 9 months of injury had poor cognitive performance (<5 percentile), moderately disabled with 7–8 concussive symptoms. The patients were subjected to 20 sessions of electroencephalogram (EEG) neurofeedback therapy during which pre- and post-MRI scan and clinical parameters were documented. The study reported that emergence of fractional anisotropy value of thalamocortical connections had significant association with clinical improvements.[8]
A study on single severe TBI patient reported progress from coma (day 8) to minimally conscious state (day 44) to posttraumatic confusional state (day 198) to fully community integration (day 366) was significantly associated with an increase in apparent diffusion coefficient and decrease in anisotropy value of thalamus.[22] In 2007, a severe TBI patient with minimal conscious state was subjected to CT-DBS, 6.5 years after injury. The patient showed improvements in behavioral, arousal, and motor function, that is, exclusively due to broad recruitment of frontal-striatal resource with thalamic stimulation.[15]
Central thalamic stimulation is directly involved with improvement in posttraumatic dystonia,[32] posttraumatic tremors,[33] and posttraumatic epilepsy.[34]
Indirect evidence
A single severe TBI patient with persistent right-sided sensory motor deficit and difficulty in maintaining both static/dynamic balance, 43 months following injury was given intermittent theta burst stimulation to cerebellum and neuromotor training for 3 weeks. The patient showed a significant improvement in right-sided limb sensation and balance while walking. The improvement was due to enforcement of cerebello-thalamo-cerebral circuit.[24]
In a study, the diffusion-tensor tractography revealed that the ascending reticular activating systems (connects pons and thalamus) tract volume and fractional anisotropy value increased significantly at 40 months as compared to 4 months was associated with patient Glasgow coma scale score improvement from 3 to 15.[23] In mild TBIs, without any intervention, majority of patients recovers where thalamus plays a major role by means of adaptive neuroplasticity.[82131]
In moderate to severe TBIs, specific neurorehabilitation helps in recovery,[152224] where thalamus has a significant role to play. The thalamus with its broad connections has a potential part in recovery of insult brain.[8152122232431] Therefore, thalamus has a key role in posttraumatic neuroplasticity.
Future directions
One of the challenges in TBI neuroplasticity is lack of quantitative evidence. If there was an objective metric that could be tracked over time in any severity of injury, TBI patients would be benefited. The longitudinal studies in all severities of brain injuries should focus and evaluate thalamus and its network's specific role in postinjury phase. These studies may give hint to by what threshold of preserved thalamic connections will enhance and facilitate recovery process and what specific rehabilitation and what time to be given. TBI neurorehabilitation should mainly focus on thalamic nuclei activation. For example, in mild to moderate, the noninvasive neurorehabilitation can be initiated like EEG neurofeedback therapy that specifically activates thalamic rhythm. Among severe TBI with minimal conscious state, CT-DBS mode may be beneficial.
CONCLUSION
Not much is known about the basics of neuroplasticity of TBI and brain structures involved in it. Our review provides evidence that thalamus is naturally involved in recovery process as in mild TBIs. Few studies from available knowledge on pathophysiological process and brain structures involved in brain injury recovery mechanisms are targeting thalamus by means of activating thalamic nuclei by DBS, a specific neurorehabilitation that targets in activating rhythm of thalamus and by metabolites that targets and activates thalamus. By activating thalamus, it refines global network connections as a result there are significant improvements in posttraumatic symptoms. Postinjury neuroplasticity may be coordinated by many brain structures; however, among them, we emphasize that thalamus plays a major key role.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The adaptive brain: A neurophysiological perspective. Prog Neurobiol. 2010;91:55-67.
- [Google Scholar]
- Neocortical dynamics: Implications for understanding the role of neurofeedback and related techniques for the enhancement of attention. Appl Psychophysiol Biofeedback. 1997;22:111-26.
- [Google Scholar]
- Recovery after brain injury: Mechanisms and principles. Front Hum Neurosci. 2013;7:887.
- [Google Scholar]
- Clinical outcomes after traumatic brain injury. Curr Neurol Neurosci Rep. 2016;16:52.
- [Google Scholar]
- The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861-8.
- [Google Scholar]
- Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321-5.
- [Google Scholar]
- Role of the thalamus in natural recovery of cognitive impairment in patients with mild traumatic brain injury. Brain Inj. 2016;30:388-92.
- [Google Scholar]
- EEG Neurofeedback therapy: Can it attenuate brain changes in TBI? NeuroRehabilitation. 2014;35:481-4.
- [Google Scholar]
- Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790-1.
- [Google Scholar]
- Frequency-selective control of cortical and subcortical networks by central thalamus. Elife. 2015;4:e09215.
- [Google Scholar]
- Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105-18.
- [Google Scholar]
- Brain activity relating to the contingent negative variation: An fMRI investigation. Neuroimage. 2004;21:1232-41.
- [Google Scholar]
- The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107-40.
- [Google Scholar]
- Advances in understanding mechanisms of thalamic relays in cognition and behavior. J Neurosci. 2014;34:15340-6.
- [Google Scholar]
- Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600-3.
- [Google Scholar]
- Contextual modulation of central thalamic delay-period activity: Representation of visual and saccadic goals. J Neurophysiol. 2004;91:2628-48.
- [Google Scholar]
- Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: A review of 107 PET activation studies. Neuroreport. 1998;9:R37-47.
- [Google Scholar]
- Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512-5.
- [Google Scholar]
- Mechanisms for selection of basic motor programs – Roles for the striatum and pallidum. Trends Neurosci. 2005;28:364-70.
- [Google Scholar]
- Recovery of consciousness after brain injury: A mesocircuit hypothesis. Trends Neurosci. 2010;33:1-9.
- [Google Scholar]
- Thalamic functional connectivity in mild traumatic brain injury: Longitudinal associations with patient-reported outcomes and neuropsychological tests. Arch Phys Med Rehabil. 2016;97:1254-61.
- [Google Scholar]
- Unexpected recovery of function after severe traumatic brain injury: The limits of early neuroimaging-based outcome prediction. Neurocrit Care. 2013;19:364-75.
- [Google Scholar]
- Recovery of injured lower portion of the ascending reticular activating system in a patient with traumatic brain injury. Am J Phys Med Rehabil. 2015;94:250-3.
- [Google Scholar]
- Clinical effects of non-invasive cerebellar magnetic stimulation treatment combined with neuromotor rehabilitation in traumatic brain injury. A single case study. Funct Neurol. 2016;31:117-20.
- [Google Scholar]
- Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A. 2006;103:17007-12.
- [Google Scholar]
- Temporally-patterned deep brain stimulation in a mouse model of multiple traumatic brain injury. Behav Brain Res. 2014;273:123-32.
- [Google Scholar]
- Monitoring functional impairment and recovery after traumatic brain injury in rats by FMRI. J Neurotrauma. 2013;30:546-56.
- [Google Scholar]
- Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22:19-31.
- [Google Scholar]
- Gating of attentional effort through the central thalamus. J Neurophysiol. 2013;109:1152-63.
- [Google Scholar]
- The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123(Pt 7):1327-38.
- [Google Scholar]
- Role of the thalamus in natural recovery of cognitive impairment in patients with mild traumatic brain injury. Brain Inj. 2015;30:1-5.
- [Google Scholar]
- Deep brain stimulation for secondary dystonia: Results in 8 patients. Acta Neurochir (Wien). 2009;151:473-8.
- [Google Scholar]
- A case of posttraumatic tremor treated by chronic stimulation of the thalamus. Mov Disord. 1993;8:206-8.
- [Google Scholar]






