Translate this page into:
Indigenous Inexpensive Practice Models for Skill Development in Neuroendoscopy
Address for correspondence: Prof. Yad Ram Yadav, Department of Neurosurgery, NSCB Medical College, Jabalpur, Madhya Pradesh, India. E-mail: yadavyr@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Neurosurgery is a branch having a tough learning curve. Residents generally get very less hands-on exposure for advanced procedures like neuroendoscopy. With the limited number of cadavers available and ethical issues associated with animal models, practice models, and simulators are becoming the able alternative. Most of these simulators are very costly. We tried to build indigenous inexpensive practice models that can help in developing most of the skills of neuroendoscopy.
Materials and Methods:
Models were built for learning hand-eye coordination, dexterity, instrument manipulation, cutting, fine dissection, keyhole concept, drilling, and simulation of laminectomy and ligamentum flavum resection. These were shown in the neuroendoscopic fellowship program conducted in authors' institute, and trainees' responses were recorded.
Results:
Both novice and experienced neuroendoscopic surgeons validated the models. There was no significant difference between their responses (P = 0.791).
Conclusion:
Indigenous innovative models can be used to learn and teach neuroendoscopic skills. The presented models were reliable, valid, eco-friendly, highly cost-effective, portable, easily made and can be kept in one's chamber for practicing.
Keywords
Clinical skills
educational models
neuroendoscopy
neurological model
simulation training
INTRODUCTION
Neuroendoscopy has become an important tool and is progressively increasingly used in cranial,[12] spinal,[345] and skull base[67] pathologies. One can use neuroendoscopy either as a solo modality in neurosurgery, such as endoscopic third ventriculostomy,[8] cerebrospinal fluid rhinorrhea,[9] pituitary surgeries,[7] craniopharyngioma,[10] colloid cyst,[2] hematoma evacuations, odontoid resection,[4] trigeminal neuralgia,[11] and surgery with the help of tubular retractor.[12] Neuroendoscopic techniques are associated with a tough learning curve. Young neurosurgeons and residents generally get very less hands-on exposure for neuroendoscopy. Cadavers remain the gold standard for learning, but there is a limit for these gifts to the medical science. Animal models have the ethical debate associated with them.[13] With this scenario, practice models and simulators prove to be an indispensable tool for learning and to prevent patient harm.[14] Learning neuroendoscopy involves an understanding of depth perception, dissection, cutting, suturing, drilling, avoiding blind spot, avoiding collision of instruments, and achieving hemostasis under two-dimensional (2D) vision.[15] At present, practice models in neuroendoscopy are quite costly[16] and out of reach for most of the young neurosurgeons. A fully-fledged laboratory may also be out of reach for some of the institutions. To circumvent this problem, we tried to build certain inexpensive models, which can cover most aspects of technicalities of neuroendoscopy and can be made or purchased by anyone.
MATERIALS AND METHODS
We chose custom-made clay utensils of inner diameter 9 cm, and a height of 9 cm, having a single hole of 3 cm on top of it, as shown in Figure 1. One had to pass a 4 mm × 18 cm size telescope (Karlstorz Inc., Tuttlingen, Germany) and instruments, and perform exercises of hand-eye coordination, dexterity, instrument manipulation, and simulation of laminectomy and ligamentum flavum removal on the foam-based floor. One exercise of drilling and an exercise of using tubular retractor for dissection and keyhole concept was also kept.
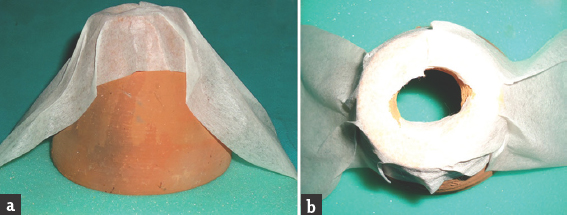
- (a) Side view and (b) top view of clay made utensil
The authors used these models in the neuroendoscopy fellowship program performed at their institute in April and September 2016. Totally, 66 post MCh/DNB neurosurgeons performed different exercises on these models under the guidance of expert faculty. In the end, these trainees were asked to rate their learning and simulations on a Likert scale of 1–5, in which 1 meant very poor, and 5 meaning excellent. We considered ratings 4 and above as a favorable response. After getting the responses, we divided the trainees according to their years of neuroendoscopic experience into two categories Group A (up to 3 years of experience) and Group B (more than 3 years of experience) and analyzed data between them. SPSS 19.0 software (SPSS Inc. An IBM Company, Armonk, New York, USA) was used, and Chi-square test was used to derivate the P value with the statistical significance considered when it was <0.05.
Exercise 1: “The hand-eye coordination, dexterity, and instrument manipulation model:” We placed a sheet in which nine squares each of 2 cm × 2 cm size were drawn and numbered, and planted pins at their intersections, as shown in Figure 2. Surgeons were asked to pass the scope and endoscopic forceps through the top hole and to shift a piece of the object between the squares without touching the pins. The second exercise in the same model was to pass a rubber band ring between the different pins.

- The hand-eye coordination, dexterity, and instrument manipulation model
Exercise 2: “The laminectomy model:” We placed an ice-cream wooden spoon on the foam floor, marked it with a sketch pen to improve precision, and stuck it with plaster. Trainees were asked to use a Kerrison punch, and punch between the lines, as shown in Figure 3.
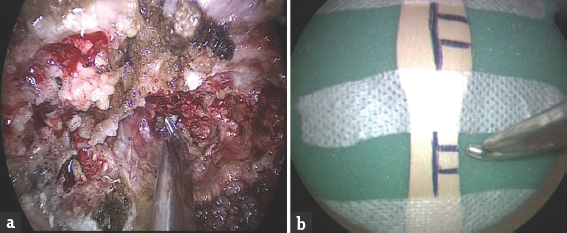
- The laminectomy model. (a) Shows the actual surgery, and (b) shows the model
Exercise 3: “The ligamentum flavum removal model:” A Foley's catheter, representing dural sac, was passed through the foam representing ligamentum flavum in this model. They were asked to separate the foam from the underlying tube by putting a patty between them and then pull the foam with a punch without injuring the tube, as shown in Figure 4.
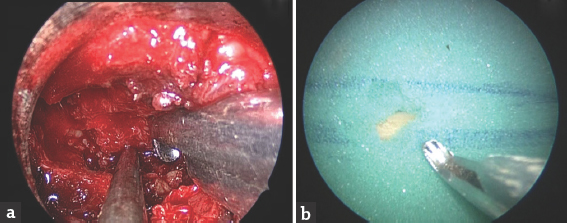
- The ligamentum flavum removal model. (a) Shows the actual surgery, and (b) shows the model
Exercise 4: “The drilling model:” We placed a raw egg between two pieces of foam and stuck it with plaster. A hole of about 1 cm on top of the foam was made, and Destandau system was inserted, as shown in Figure 5. The trainees were asked to pass the scope, the suction, and a drill through their respective channels and then drill the egg, and write his/her name on the egg by slow paint brush technique.
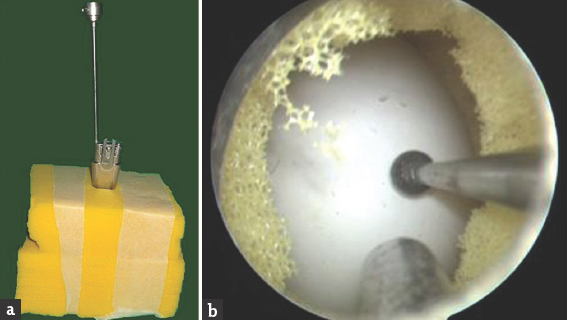
- The drilling model. (a) Shows the outside, and (b) shows the endoscopic view of the model
Exercise 5: “Dissection using tubular retractor model:” We placed a thick piece of foam over a prior cut papaya having boundaries marked inside it with pins. From the top of the foam a tubular retractor, described by Yadav et al.[12] was passed. The telescope was fixed in the holder, and instruments were used to perform dissection and cutting. Initially, the tubular retractor was kept vertical, and exercise was done, and later it was tilted to work in a wide area to simulate minimally invasive surgery. Figure 6 shows this model.
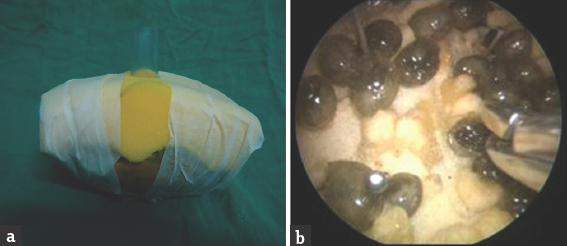
- Dissection using tubular retractor model – (a) shows the outside, and (b) shows the endoscopic view of the model
Exercise 6: “The cutting model:” A piece of glove was pinned to the foam, and a square was marked over it. Fellows were asked to make the initial incision with a knife and then cut that glove with the endoscopic scissor without injuring the underlying foam, as shown in Figure 7.
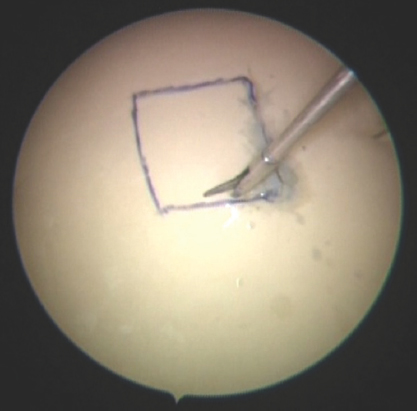
- Cutting model - it shows the line drawn on the glove, which is pinned on the foam
Fellows were taught the disadvantages of keeping instrument, scope and the surgical target in straight line and the importance of triangularization in avoiding blind spot, avoiding tissue to pull toward scope, technique of hand support, precision grip, etc.
RESULTS
Sixty-six trainees gave their ranking for these models. Totally, 50 (75.8%) responses gave four and above ratings as shown in Figure 8.
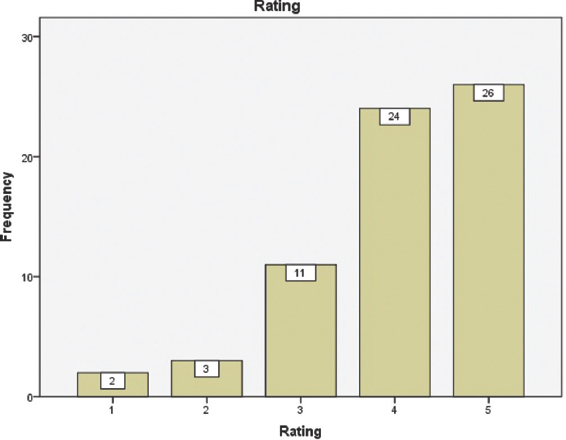
- Trainees' responses
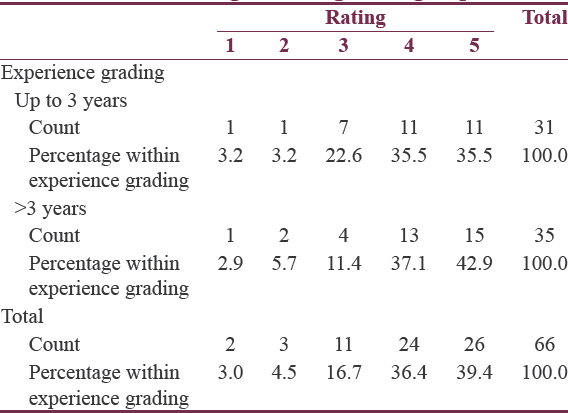
Experience wise: Group A was neurosurgeons up to 3 years of experience (n = 31, i.e., 47%), and Group B was more than 3 years (n = 35, i.e., 53%). Group wise also the trainees gave a favorable response as shown in Table 1. Statistically, there was no significant difference between the responses with the P = 0.791.
DISCUSSION
At present, with increasing lawsuits and high expectations from patients, the residents are getting less and less hands-on exposure in neurosurgery. The scenario is even worse for neuroendoscopy. With less number of cadavers available and ethical and validity debate associated with animal models, simulators, and hands on models provide an able alternative. The existing good simulators and practice models around the world are either too costly[14] such as S.I.M.O.N.T. neurotrainer (Prodelphus, Germany), Phacon sinus system (Phacon, Leipzig, Germany), and Kezlex endoscopic models (Ono and Co., Tokyo, Japan), or provide only the basic training of hand-eye coordination.[17] The presented models provided good training of hand-eye coordination, instrument manipulation, dexterity, fine dissection, cutting, drilling, and simulation of laminectomies and removal of ligamentum flavum. These models were also used to teach the basic concepts of neuroendoscopy. These models were reliable, inexpensive (built up cost of <50 rupees each), easy to build, have low maintenance, nontoxic, and can be placed in one's chamber for practicing.
The results showed that there was an equal benefit to both novice and experienced neuroendoscopic surgeons, wanting to learn, and improve skills. For a neurosurgeon, who knows the anatomy, learning neuroendoscopy needs the skills of hand-eye coordination, dissection, cutting, suturing, drilling, and hemostasis under 2D vision. Our models provided good training for the said skills, barring hemostasis. One can use a wired camera instead of the conventional endoscope for practice, which can further reduce the costs. A webcam can also be put to obviate the need of camera source, as is used by Espinoza et al.,[18] but that will take away the liberty of zooming and recentering of the target from the surgeon. One can use his laptop or a simple television in place of monitor to practice. Led light source can be used for illumination.
However, the presented models had certain limitations also. First, they lacked the exercise for hemostasis, and second, the simulations for nasal and ventricular anatomy were not made as in company made ones.[19] We could have kept separate ratings for individual exercises to better assess each model. Finally, the good ratings given by trainee fellows may be because of goodwill effect as they had come for the fellowship program. Therefore, we also need more validation of these models by other practitioners of neuroendoscopy.
CONCLUSION
Indigenous innovative models can be used to learn and teach neuroendoscopic skills. The presented models were reliable, valid, eco-friendly, inexpensive, portable, easily made, and can be kept in one's chamber for practicing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Fully endoscopic supraorbital keyhole approach to the anterior cranial base: A cadaveric study. J Neurosci Rural Pract. 2015;6:361-8.
- [Google Scholar]
- Neuroendoscopic treatment of idiopathic occlusion of unilateral foramen of Monro presenting as chronic headache. J Neurosci Rural Pract. 2016;7:128-30.
- [Google Scholar]
- Clinical outcomes and efficacy of transforaminal lumbar endoscopic discectomy. J Neurosci Rural Pract. 2015;6:344-8.
- [Google Scholar]
- Endoscopic transoral excision of odontoid process in irreducible atlantoaxial dislocation: Our experience of 34 patients. J Neurol Surg A Cent Eur Neurosurg. 2013;74:162-7.
- [Google Scholar]
- Endoscopic anterior decompression in cervical disc disease. Neurol India. 2014;62:417-22.
- [Google Scholar]
- Purely endoscopic pterional extradural approach: A novel technique for repair of cerebrospinal fluid rhinorrhea. J Neurosci Rural Pract. 2016;7:310-3.
- [Google Scholar]
- Endoscopic endonasal trans-sphenoid surgery of pituitary adenoma. J Neurosci Rural Pract. 2012;3:328-37.
- [Google Scholar]
- Endoscopic management of cerebrospinal fluid rhinorrhea. Asian J Neurosurg. 2016;11:183-93.
- [Google Scholar]
- Endoscopic endonasal trans-sphenoid management of craniopharyngiomas. Asian J Neurosurg. 2015;10:10-6.
- [Google Scholar]
- Endoscopic vascular decompression in trigeminal neuralgia? Turk Neurosurg 2016 doi: 10.5137/1019-5149.JTN.17046-16.1. [Epub ahead of print]
- [Google Scholar]
- A new minimally invasive tubular brain retractor system for surgery of deep intracerebral hematoma. Neurol India. 2011;59:74-7.
- [Google Scholar]
- Neurosurgery simulation in residency training: Feasibility, cost, and educational benefit. Neurosurgery. 2013;73(Suppl 1):39-45.
- [Google Scholar]
- Complication avoidance and its management in endoscopic neurosurgery. Neurol India. 2013;61:217-25.
- [Google Scholar]
- Ventricular and skull base neuroendoscopy simulation in residency training: Feasibility, cost, and resident feedback. J Surg Simul. 2014;1:22-9.
- [Google Scholar]
- Training to acquire psychomotor skills for endoscopic endonasal surgery using a personal webcam trainer. J Neurosurg. 2013;118:1120-6.
- [Google Scholar]
- PsT1: A Low-cost optical simulator for psychomotor skills training in neuroendoscopy. World Neurosurg. 2015;83:1074-9.
- [Google Scholar]
- Quality assessment of a new surgical simulator for neuroendoscopic training. Neurosurg Focus. 2011;30:E17.
- [Google Scholar]






