Translate this page into:
Efficacy of Botulinum Toxin Type A in Trigeminal Neuralgia in a South Asian Cohort
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The antinociceptive effect of botulinum toxin-A (BTX-A) in trigeminal neuralgia (TN) has been described. We evaluated effects of BTX-A in relieving pain in patients with refractory TN at National Hospital of Sri Lanka.
Materials and Methods:
Pain in patients with TN was assessed using a visual analog from 0 to 10. Three months after commencement of drug therapy with ≥2 drugs including one first-line drug (carbamazepine/oxcarbazepine), pain scores were re-assessed. Twenty-two patients who did not report improvement of ≥50% at 90 days’ posttreatment were recruited. They were given adjunct BTX-A directly to the trigger point (if identified) or intradermal. Pain scores were assessed at 10, 20, 30, 60, and 90 days’ posttreatment.
Results:
There was a statistically significant improvement in mean pain scores at 10, 20, 30, 60, and 90 days’ posttreatment (5.59 [standard deviation (SD) = 2.7], 5.68 [SD = 2.6], 5.27 [SD = 3.2], 4.77 [SD = 3.7], and 5.32 [SD = 4.0]) compared to pre-BTX-A treatment (7.14, SD = 2.2). Percentage reduction in mean pain score ranged from 20.4% to 33.1%. Maximum response was at day 60 post-BTX-A (50% had ≥50% reduction in pain). No significant difference was found in response with higher doses and injection strategy.
Conclusion:
Consistent statistically significant reductions in pain scores at the aforesaid intervals compared to pretreatment means that there is a place for BTX in refractory TN.
Keywords
Efficacy of botulinum toxin in trigeminal neuralgia
nonpharmacological treatment of trigeminal neuralgia
refractory trigeminal neuralgia
INTRODUCTION
Trigeminal neuralgia (TN) is a common and potentially disabling pain syndrome.[1] TN is likely to have an exacerbating and remitting course. Over time, pain-free intervals appear to diminish, and pain becomes progressively more medically intractable. Temporary spontaneous remission may occur at any time.
The treatment of TN includes both pharmacological and nonpharmacological methods. Carbamazepine and oxcarbazepine are considered first-line therapy.[2] In addition, gabapentin,[3] lamotrigine,[4] phenytoin,[5] and non-antiepileptic drugs such as baclofen are also used although evidence is insufficient.[6]
Nonpharmacological methods include surgery,[7] gamma knife therapy,[8] and percutaneous procedures.[9]
Approximately 25%–50% of patients eventually stop responding to drug therapy and require alternative treatment, often nonpharmacological treatment.[10]
Botulinum toxin-A (BTX-A) is reported to be effective in the treatment of migraine and myofascial pain syndrome including TN.[11] BTX-A produces its antinociceptive effect in TN by several proposed mechanisms. BTX-A may reduce neurogenic inflammation by inhibiting the release of glutamate, substance P, and calcitonin gene-related peptide (CGRP) in the sensory terminal.[11] It may also reduce the release of substancePin dorsal root ganglia[12] and inhibit CGRP release in trigeminal sensory neurons of the brainstem.[13]
Only a handful of studies have been done to establish the efficacy and duration of effect in TN. The dearth of data is especially true in the South Asian population. In this study, we assessed the efficacy and duration of pain relief following BTX-A injection for medically refractory TN in a South Asian cohort.
MATERIALS AND METHODS
This was an observational study done over 6 months. Patients presenting between October 1, 2012, and April 1, 2013, with TN (a disorder characterized by recurrent unilateral brief electric shock-like pains, abrupt in onset and termination, limited to the distribution of one or more divisions of the trigeminal nerve, and triggered by innocuous stimuli. Patients without an apparent cause were included in the study. There may or may not be, additionally, persistent background facial pain of moderate intensity) diagnosed according to the International Classification of Headache Disorders-2[14] (ICHD-2) to neurology clinics at National Hospital of Sri Lanka, Colombo, Sri Lanka, were assessed using a 10-point Likert scale (visual analog scale [VAS]) to assess pain with 11 points on a ruler of a 10 cm length where 0 denoted “no pain” and 10 denoted “severe pain”. After commencement of drug therapy with ≥2 drugs including one first-line drug (carbamazepine/oxcarbazepine), pain scores were re-assessed at intervals of 10, 20, 30, 60, and 90 days. Sample size calculated with an anticipated effect size (Cohen's d) of 0.80 and a desired statistical power level of 0.80 and a probability level of 0.05. Required minimum sample size for a one-tailed hypothesis is 42 (minimum sample size per group: 21).
Patients who had a pain score of ≥6 and who did not report improvement of ≥50% after 90 days of treatment were recruited. Patients having another disorder associated with facial pain coexisting or independent of TN according to ICHD-2 criteria, patients whose pain was effectively controlled by methods other than BTX-A, patients who refused consent to take part in the study, patients with hypersensitivity reaction to botulinum toxin or human albumin, patients with neurological diseases such as multiple sclerosis, myasthenia gravis, and Eaton–Lambert syndrome,[15] and patients under 12 years of age were excluded from the study.
Recruited participants were given BTX-A (adjunct to medical therapy) directly to the trigger point (if identified and technically feasible) or intradermal in the distribution of the pain. Pain scores were assessed at intervals of 10, 20, 30, 60, and 90 days’ posttreatment using the 10-point Likert scale (0 denoted “no pain” and 10 denoted “severe pain”).
Demographic data, comorbidities, disease duration, dosage, site of injection, and major side effects affecting quality of life (transient facial asymmetry during dynamic movements, erythema, and edema of skin at injection site affecting quality of life[15]) were recorded in each participant. Periodical assessment of pain scores (i.e. intervals of 10, 20, 30, 60, and 90 days) was presented as mean pain score with the standard deviation (SD). Periodically assessed pain scores were compared with the pre-BTX-A treatment pain scores, using Student's one-sided paired t-test. Percentage reduction of mean pain scores was calculated for the aforesaid time intervals in both drug therapy alone and with adjunct BTX-A and compared. The number of patients reporting improvement in pain scores and the number reporting worsening of pain scores were recorded with BTX-A. Mean pain scores of patients given higher doses of BTX-A (>25 mg) at the said intervals were compared with mean pain scores of patients who received lower doses (≤25 mg) using the Mann–Whitney U-test (nonparametric statistical method). Furthermore, mean pain scores of patients in whom a trigger point was identified and injected were compared with patients in whom a trigger point was not identified using the Mann–Whitney U-test. P < 0.05 was considered statistically significant in all instances. Data were analyzed using SPSS version 21 software.
Informed written consent was obtained from all participants. Ethical clearance for the study was obtained from the Ethics Review Committee of the Faculty of Medicine, University of Colombo, Sri Lanka.
RESULTS
Twenty-two patients were recruited into the study. Mean age was 55.86 SD ± 8.72 years. Twelve (54.5%) were males. The comorbidities in the cohort included hypertension (n = 2; 9.1%) and bronchial asthma (n = 2; 9.1%). The mean duration of the disease before recruitment was 6.88 SD ± 5.49 years. Trigger point was identified and injected in 15 (68.2%) participants. Doses used ranged from 15 to 50 IU (mean = 28.9 IU).
Before the drug treatment, mean pain score was 7.41 (SD = 2.3). Mean scores for pain at intervals of 10, 20, 30, 60, and 90 days’ postdrug treatment were 6.86 (SD = 1.9), 6.59 (SD = 1.8), 6.55 (SD = 1.9), 6.59 (SD = 1.9), and 6.59 (SD = 1.8), respectively.
Pre-BTX-A (adjunct) treatment mean pain score was 7.14 (SD = 2.2). Mean scores for pain at intervals of 10, 20, 30, 60, and 90 days’ posttreatment were 5.59 (SD = 2.7), 5.68 (SD = 2.6), 5.27 (SD = 3.2), 4.77 (SD = 3.7), and 5.32 (SD = 4.0), respectively [Table 1]. None were pain free during the study period.
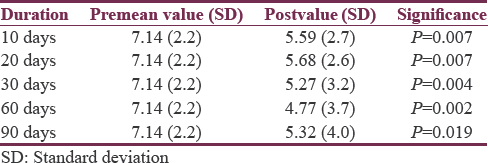
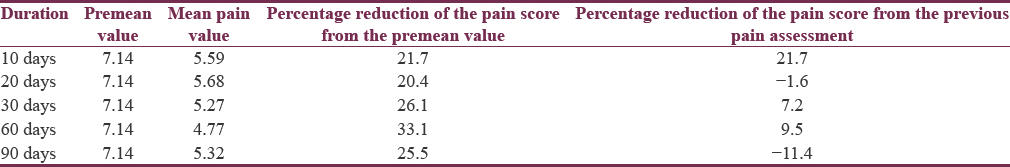
The percentage reduction in mean pain score after injecting BTX-A ranged from 20.4% to 33.1% with a maximum response seen at 60 days following treatment [Table 2]. There was a slight reduction to 25.5% thereafter at 90 days. This finding was further strengthened with 4 (18.2%), 5 (22.7%), 10 (45.5%), 11 (50%), and 9 (40.9%) participants experiencing ≥50% reduction in pain scores at epochs of 10, 20, 30, 60, and 90 days, respectively.
Participants reported reduction in pain scores (12/22 [54.5%] –14/22 [63.6%]) throughout the 90 days’ follow-up period. The maximum number of participants (n = 06, 27.3%) who had reported worsening of pain scores was at day 90 [Table 3].

There was no statistical difference in pain scores in patients receiving higher doses of BTX-A versus lower doses across all time intervals [Table 4]. There was also no significant difference to response in pain scores in participants whose trigger point was injected compared to participants in whom other sites were injected across all time intervals [Table 5].


The mean percentage reduction in pain scores after drug use alone and the mean percentage reduction of pain scores after adjunct BTX-A were compared at equivalent time points [Table 6]. The mean percentage reduction in pain scores after drug use alone ranged from 4.09% to 6.82%, while after BTX-A, the range had increased to 20.00%–36.36%. Statistically significant differences were observed at days 10.30 and 60 in mean percentage reduction in pain scores after drug use alone and after BTX-A as adjunct therapy [Table 6]. Days 20 and 90 posttreatment did not show significant differences in both groups.

Treatments were generally tolerated, no systemic reactions were noted, and there were no serious injection-related adverse events affecting the quality of life of the patients.
DISCUSSION
By directly inhibiting the release of peripheral neurotransmitters, BTX-A is able to directly inhibit peripheral sensitization and indirectly inhibit central sensitization to pain.[16] Our study demonstrated sustained improvement in pain scores compared to pretreatment baseline throughout the follow-up period of 90 days. We also found statistical differences in the mean percentage reduction in pain scores between drug therapy alone and BTX-A as adjunct therapy at days 20, 30, and 60 with better response to BTX-A adjunct therapy, while the mean percentage reduction in pain was not statistically significant at days 10 and 90. Inadequate numbers in the cohort could have contributed to this result.
The therapeutic effect of BTX-A in TN was first mentioned by Wang and Jankovic.[17] Thereafter, a few studies have reported efficacy with BTX-A. A double-blind, randomized, and placebo-controlled study by Wu et al.[18] included 42 patients with TN according to the ICHD-2 criteria,[14] who failed to respond to conventional treatment (patients with a mean VAS score ≥4, mean attack frequency ≥4 per day). The follow-up period was 12 weeks. A total of 75 units of BTX-A were injected in divided proportions along the area of pain distribution. The primary end point was assessed by the proportion of responders, defined as patients with at least 50% reduction in frequency and/or intensity of pain. This study found 68.18% of patients in the experimental group to be responders as compared to 15.00% in the placebo group. The difference was statistically significant. Another open labeled study by Piovesan et al.,[19] found that all 13 patients showed improvement; 4 patients remained pain free, and 9 patients reported partial pain relief with >50% reduction in medication usage. The beneficial effect lasted for approximately 60 days. An open-label trial by Li et al.[20] reported 100% (88/88) responder rates at 2 months. In a systematic review,[21] which looked into 6 studies and included a total of 101 patients, response to BTX-A was achieved in approximately 70%–100% of patients. A network meta-analysis by Sridharan and Sivaramakrishnan[22] also reiterated the above findings by reporting two studies with at least 50% reduction in pain scores while one reported complete pain relief. We found that, in our study, 54.5% (maximum of 63.6% at days 30 and 60 following BTX-A) of our patients had improved pain scores at the end of 3 months.
The above studies quote a high proportion of responders in their respective cohorts. This kind of efficacy was not observed in our cohort with a maximum of 50% achieving ≥50% reduction in pain scores at day 60 post-BTX-A. Reasons for this could be varied and may be due to differences in cohort characteristics. Difference in definition of our cohort may have caused this disparity. We recruited patients who had pain scores of ≥6 and who did not show ≥50% reduction with at least 2 drugs while Wu et al. recruited participants with pain scores of ≥4 resistant to treatment. Resistance to treatment was not defined in this cohort. Moreover, Wu et al. had also looked into the frequency of attacks while our study did not.
Differences in dosage may also have contributed to the above disparity. In the study by Wu, a total of 75 units of BTX-A were injected in divided proportions along the area of pain distribution to all participants. The dose varied in our cohort and ranged from 15 to 50 IU with a mean of 28 IU. A double-blinded randomized control study by Shehata et al.[23] studied 20 participants with intractable idiopathic TN, which they defined as a failure to achieve a 50% relief in pain intensity quantified by VAS and/or the frequency of paroxysms during the previous 3 months which was similar to the selection criteria in our study though we had further defined the minimum number of drugs necessary to be defined as “drug resistant” which Shehata et al. had not. The active treatment arm consisted of 10 patients who received 0.1 mL of BTX-A (5 U/0.1 mL) applied subcutaneously per point (total dose ranged from 40 to 60 U). After 12 weeks, statistically significant reductions in VAS scores (a decrease of 6.5 compared with 0.3 for saline) were observed with greater reductions in pain scores seen compared to our study. Again in the aforesaid study, higher doses were used compared to ours. We must also emphasize on the fact that variability in dosing, the number of injection sites, and injecting strategy (intradermal vs. trigger point) could have contributed to lower response to BTX-A when compared to other studies. However, we observed in our study that higher doses compared to lower doses as well as injection strategy did not result in a statistically significant difference in response. This may be the result of low numbers in each group. It is interesting to also note that the study by Piovesan et al.,[19] found that 6-9 U of BTX-A induced significant decreases in pain intensity and decreases in the area of pain, suggesting that lower doses are also feasible. Furthermore, Zhang et al.[24] studied the effects of different dosages of BTX-A in 84 patients with TN who had failed to respond to recent treatment (mean pain intensity score ≥4, mean attack frequency ≥4/day; course >4 months) and were randomized to receive saline, 25 U/mL or 75 U/mL of BTX-A intradermally and/or submucosally (total doses were divided by 20 and applied at 20 points). VAS scores of both BTX-A groups were lower than in the placebo group from the first to the 8 weeks (P < 0.017), and there was no difference between the 25 U and 75 U groups. There was also no difference in number of responders between the groups that received different doses strengthening the premise that the effect of BTX-A may not be dose dependent.
Another variable which could explain lower efficacy in our study is the number of injection sites. The number of injections sites varied from patient to patient in our study. In Wu's study, injection was done at 15 sites. However, in a randomized open-ended study by Türk et al.,[25] in which 8 patients were injected with 100 units of BTX-A at only 2 sites, concluded that BTX-A can be utilized in cases of refractory TN.
A major determinant of refractory TN is the duration of the disease. Hence, longer the duration of TN there is a higher chance of TN being refractory to therapy. Our cohort had a mean duration of disease of 6.88 years which was longer than studies by Shehata et al.[23] (5.33 years), Wu et al.[18] (5.92 years), and Zhang et al.[24] (5.97 years). This also may explain the lower number of responders in our cohort compared to other quoted studies.
BTX-A has a fast onset of action with its significant effect reaching within 1–2 weeks of injection and maximum effect within 4–6 weeks.[21] This was also seen in our study with effect seen within 10 days. However, maximum effect in our study was observed in 60 days.
Confounders to our results need highlighting. The effect BTX-A in our cohort may be explained by the placebo effect. The placebo effect of BTX-A has been described for primary headaches. In a large placebo-controlled dose-finding European trial of BTX-A for migraine, 25%–55% of the placebo patients experienced a decrease of at least 50% in migraine frequency.[26] In trials of BTX-A for chronic daily headache, placebo responders rated the improvement from 21% to 23.4%.[2728]
Natural variations in pain intensity in TN may contribute to the improvements in pain scores in our cohort as the pain in TN can have an exacerbating and remitting course.
The use of BTX-A as a therapeutic option for treating TN has several drawbacks: the method is operator-dependent; there are no consensus guidelines; and “refractory trigeminal neuralgia” is a concept that has yet to be defined.[29]
However, our study suggests that BTX-A treatment may provide a clinically significant benefit to refractory TN in adults. The effect is rapidly achieved, within 10 days. BTX-A treatment seems to be well tolerated with minimal systemic adverse events. Therefore, it represents a promising treatment of TN with favorable risk-to-benefit ratio. However, a well-designed randomized, controlled, double-blinded trial is required to investigate the optimal dose of BTX-A treatment and the duration of therapeutic efficacy.
CONCLUSION
Consistent statistically significant reductions in pain scores at the aforesaid intervals compared to pretreatment means that there is a place for BTX in refractory TN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Medical management of trigeminal neuropathic pains. Expert Opin Pharmacother. 2010;11:1239-54.
- [Google Scholar]
- Gabapentin for idiopathic trigeminal neuralgia: Report of two cases. Neurology. 1997;48:1467.
- [Google Scholar]
- Clinical effectiveness of lamotrigine and plasma levels in essential and symptomatic trigeminal neuralgia. Neurology. 1997;48:1714-7.
- [Google Scholar]
- Non-antiepileptic drugs for trigeminal neuralgia. Cochrane Database Syst Rev. 2006;19:CD004029.
- [Google Scholar]
- Various surgical modalities for trigeminal neuralgia: Literature study of respective long-term outcomes. Acta Neurochir (Wien). 2008;150:243-55.
- [Google Scholar]
- Percutaneous retrogasserian glycerol rhizolysis for treatment of trigeminal neuralgia. Technique and results in 191 patients. J Neurosurg Sci. 1995;39:37-45.
- [Google Scholar]
- The use of botulinum toxin for the treatment of chronic facial pain. J Pain. 2002;3:21-7.
- [Google Scholar]
- Presynaptic effects of botulinum toxin type A on the neuronally evoked response of albino and pigmented rabbit iris sphincter and dilator muscles. Jpn J Ophthalmol. 2000;44:106-9.
- [Google Scholar]
- Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009;29:4981-92.
- [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9-160.
- [Google Scholar]
- Role of botulinum toxin type-A (BTX-A) in the management of trigeminal neuralgia. Pain Res Treat 2013 2013:831094.
- [Google Scholar]
- Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785-93.
- [Google Scholar]
- Botulinum toxin type A for the treatment of trigeminal neuralgia: Results from a randomized, double-blind, placebo-controlled trial. Cephalalgia. 2012;32:443-50.
- [Google Scholar]
- An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology. 2005;65:1306-8.
- [Google Scholar]
- Therapeutic effect of botulinum toxin-A in 88 patients with trigeminal neuralgia with 14-month follow-up. J Headache Pain. 2014;15:43.
- [Google Scholar]
- Therapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: A systematic review. J Headache Pain. 2013;14:72.
- [Google Scholar]
- Interventions for refractory trigeminal neuralgia: A Bayesian mixed treatment comparison network meta-analysis of randomized controlled clinical trials? Clin Drug Investig 2017 doi: 10.1007/s40261-017-0553-9
- [Google Scholar]
- Botulinum toxin-type A: Could it be an effective treatment option in intractable trigeminal neuralgia? J Headache Pain. 2013;14:92.
- [Google Scholar]
- Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: Observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J Headache Pain. 2014;15:65.
- [Google Scholar]
- Botulinum toxin and intractable trigeminal neuralgia. Clin Neuropharmacol. 2005;28:161-2.
- [Google Scholar]
- A multicentre, double-blind, randomized, placebo-controlled, parallel group study of multiple treatments of botulinum toxin type A (BoNTA) for the prophylaxis of episodic migraine headaches. Cephalalgia. 2007;27:492-503.
- [Google Scholar]
- Botulinum toxin type A (BOTOX) for the prophylactic treatment of chronic daily headache: A randomized, double-blind, placebo-controlled trial. Headache. 2005;45:293-307.
- [Google Scholar]
- Botulinum toxin type A for the prophylactic treatment of chronic daily headache: A randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2005;80:1126-37.
- [Google Scholar]
- OnabotulinumtoxinA for trigeminal neuralgia: A review of the available data. Arq Neuropsiquiatr. 2015;73:877-84.
- [Google Scholar]






