Translate this page into:
Acute ischemic stroke and the golden hour: Critical updates
*Corresponding author: Kalyan Sarma, Department of Radiology, All India Institute of Medical Sciences, Guwahati, Assam, India. kalyansarma@aiimsguwahati.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Khandelwal A, Sarma K, Hussain M, Dikshit P, Baidya D. Acute ischemic stroke and the golden hour: Critical updates. J Neurosci Rural Pract. doi: 10.25259/JNRP_17_2025
Abstract
Acute ischemic stroke (AIS) remains a major contributor to illness and death, occurring due to the sudden occlusion of a cerebral artery, which results in brain ischemia and potential neuronal damage. The “golden hour” refers to the critical first 60 min following stroke onset, during which rapid diagnosis and treatment are essential to optimize patient outcomes. Timely thrombolysis with intravenous tissue plasminogen activator (tPA) or endovascular thrombectomy can significantly reduce infarct size and improve functional recovery. Advancements in imaging modalities such as computed tomography and magnetic resonance imaging facilitate the swift distinction between ischemic and hemorrhagic stroke, as well as the detection of viable brain tissue. Public awareness of stroke symptoms and efficient prehospital triage systems are pivotal in reducing delays in seeking medical attention. Within this narrow time window, a streamlined multidisciplinary approach is warranted to ensure rapid evaluation and treatment initiation. Despite advancements, challenges persist, including delayed recognition, limited access to specialized stroke centers, and contraindications to tPA. Efforts to improve stroke systems of care and promote public education are crucial in enhancing the number of patients treated within the golden hour. Optimizing early intervention remains the cornerstone of alleviating the global impact of AIS and improving patient prognosis.
Keywords
Golden hour
Ischemic
Stroke
Thrombolysis
Triage
INTRODUCTION
Acute ischemic stroke (AIS) is one of the leading causes of mortality and long-term disability worldwide, resulting from the sudden occlusion of a cerebral artery and subsequent loss of blood flow to brain tissue. Rapid diagnosis and timely intervention are crucial, as brain cells begin to die within minutes of oxygen deprivation. The concept of the “golden hour,” i.e., the first 60 minutes following symptom onset has emerged as a critical window for initiating treatment and improving patient outcomes. During this period, swift medical response, including neuroimaging and thrombolytic therapy, can significantly reduce neuronal damage and enhance recovery. This review highlights recent advancements in pre-hospital stroke recognition, emergency response systems, and therapeutic strategies aimed at maximizing the potential of the golden hour and improving survival and functional outcomes in AIS patients.
GLOBAL BURDEN OF STROKE
Stroke poses a significant global health burden and ranked as the second leading cause of death in 2020, resulting in 6.6 million fatalities. It was also the third leading cause of disability, accounting for 143 million disability-adjusted life-years (DALYs), following neonatal disorders in children and ischemic heart disease in adults.[1] In 2021, stroke-related deaths rose to 7.3 million, with DALYs reaching 160.5 million.[2] Further, the World Stroke Organization and the Lancet Neurology Commission report highlights that stroke-related deaths are projected to increase from 6.6 million in 2020 to a staggering 9.7 million by 2050. In addition, DALYs are anticipated to approach 190 million within the same period.[3] South East Asian countries contribute to over 40% of global stroke-related deaths, with India recording the highest mortality rate.[4]
Globally, acute ischemic stroke (AIS) constitutes 65.3% (62.4–67.7), intracerebral hemorrhage (ICH) constitutes 28.8% (28.3–28.8), and subarachnoid hemorrhage constitutes 5.8% (5.7–6.0) of incident strokes.[2] Worryingly, studies indicate a rising incidence of stroke among younger individuals, specifically those under 55 years, across the globe.[5]
CONCEPT OF GOLDEN HOUR INTRAVENOUS THROMBOLYSIS (IVT)
There is considerable evidence from multicenter trials that treatment with IVT significantly reduces stroke-related mortality and disability and improves functional outcomes in patients with AIS when administered within 3 h, or 4.5 h in selected cases.[6,7] The effectiveness of IVT is closely linked to time,[8] with the greatest benefit observed when given within the initial “golden” hour after symptom onset.[9-12] The latest meta-analysis comprising seven studies from 2015 to 2023 involving 78,826 patients found that golden hour IVT (0–60 min; n = 1,613) was associated with higher odds of achieving excellent functional outcomes at 90 days (odds ratios [OR] 1.40, 95% confidence interval [CI] 1.16–1.67) and good functional outcomes at 90 days (OR 1.38, 95% CI 1.13–1.69) compared to IVT given beyond the golden hour (61 min–4.5 h; n = 77,213). The rates of symptomatic ICH and mortality were comparable between the two groups.[13] These findings are consistent with excellent outcomes rates reported in the Safe Implementation of Treatments in Stroke-East (SITS-EAST) registry for patients receiving golden hour IVT compared with later treatment (modified Rankin scale [mRS] 0–1: 46.5% vs. 34.0%).[12] These findings support the need for intensified efforts to accelerate IVT administration within the golden hour following AIS onset.
THE RATIONALE FOR GOLDEN HOUR IVT
The higher success rates within the golden hour of IVT could be because, within the 1st h after an AIS, the reduction in cerebral blood flow is often not enough to cause irreversible damage. Rather, during the 1st h, most of the brain tissue affected by the ischemic insult is located in the penumbral region.[14] In addition, in the early period following the ischemic insult, the thrombus has a disorganized composition that facilitates thrombolysis.[10] Moreover, higher rates of recanalization have been demonstrated with golden hour IVT. Di Lorenzo et al. defined complete recanalization as a modified Treatment in Cerebral Infarction (mTICI) score of 3 and demonstrated a significant improvement with golden hour IVT (28.0% vs. 6.8%).[10] In contrast, Tsivgoulis et al. used a relative reduction of more than 40% in the National Institutes of Health Stroke Scale (NIHSS) score at 2 h after IVT as a predictor of complete recanalization, showing notable improvement with golden hour IVT (39.4% vs. 21.4%).[12]
BARRIERS TO THE WIDESPREAD IMPLEMENTATION OF GOLDEN HOUR IVT
Achieving IVT within the critical golden hour following AIS is challenging, particularly within the in-hospital settings. Despite the time-dependent benefits of IVT, <5% of patients arrive within the golden hour.[15] Data from the SITS-EAST registries and get with the guidelines-stroke have shown that golden hour IVT rates are as low as 0.8% and 1.3%, respectively.[12,14] Factors contributing to treatment delays include longer time from symptom onset to seeking medical help, late arrivals to the hospital, delays in preferential triaging, delays in performing brain imaging and its interpretation, limited access to neuroscience experts (especially in rural areas), and the management of severe hypertension which impedes IVT.[9] In India, the problem is further compounded by factors such as the low affordability of treatment costs, deficient knowledge about the early indicators of an acute stroke and the consequent need to seek urgent help, and limited dedicated stroke care centers [Table 1].[16]
| Barriers to the Widespread Implementation of Golden Hour IVT | Potential Interventions to Overcome the Barriers |
| Lack of awareness of signs and symptoms of an acute stroke. | Widespread stroke awareness programs and education campaigns should be organized, especially at the community level. Social media can be highly effective in promoting such awareness. |
| Longer time from symptom onset to seeking medical help. | Use of appropriate prehospital stroke screening tool should be emphasized for rapid identification of signs and symptoms of an acute stroke. |
| Late arrivals to the hospital. | Facilities for the rapid deployment of ambulances and response teams should be strengthened. |
| Delays in preferential triaging. | First responders in the emergency department should be trained to quickly identify the signs and symptoms of an acute stroke. |
| Delays in performing brain imaging and its interpretation. | Ensuring the availability of imaging (NCCT/MRI) and radiologists for accurate interpretation and diagnosis is crucial. |
| Limited access to neuroscience experts (especially in rural areas). | Focus on establishing a dedicated stroke team in all referral centers. |
| Failure to manage severe hypertension which impedes IVT. | A dedicated stroke team, including intensivists, should manage severe hypertension to ensure timely IVT. Availability of drugs to control severe hypertension should be ensured. |
| Low affordability of treatment costs. | Public healthcare funding, insurance coverage, subsidized stroke care programs, low-cost diagnostic tools, etc., are various means to curtail treatment costs. |
| Limited dedicated stroke care centers. | Establishing more and more dedicated stroke centers should be one of the top priorities. |
MRI: Magnetic resonance imaging, IVT: Intravenous thrombolysis, NCCT: Noncontract computed tomography
Unlike IVT, endovascular thrombectomy (EVT) is a minimally invasive, image-guided procedure designed to restore blood flow by extracting a clot or thrombus from a blocked artery. However, the modality is limited to experienced stroke centers that have facilities for performing cerebral angiography, availability of interventional neuroradiologists, and a well-equipped periprocedural care team. This necessity often limits EVT availability to comprehensive stroke centers, making rapid patient transfer a critical factor in treatment success.
RECOMMENDATIONS AND STRATEGIES FOR GOLDEN HOUR IVT
Prehospital care
Prompt recognition of stroke symptoms is crucial for obtaining timely medical care. However, awareness of stroke warning signs and risk factors remains largely poor. Stroke education campaigns should be tailored to emphasize the early recognition of stroke symptoms and the urgency of seeking immediate emergency care (class of recommendation [COR]-I, level of evidence [LOE]-B-NR).[17] Further, to reinforce stroke identification, the use of prehospital stroke screening tools by Emergency Medical Services personnel is recommended (COR-I, LOE-B-NR).[17] Delays in transport to the target hospital, preferably the closest healthcare facility able to administer recombinant tissue-type plasminogen activator (rtPA, alteplase), should be minimized through pre-arrival notification, allowing appropriate hospital resources to be mobilized before the patient’s arrival (COR-I, LOE-B-NR).[17]
Over the past few years, numerous screening tools have been introduced to facilitate the rapid identification of acute stroke in prehospital settings. These include the Cincinnati Prehospital Stroke Scale, Face, Arm, Speech, Time (FAST), Balance, Eyes, FAST (BEFAST), Los Angeles Prehospital Stroke Screen, Melbourne Ambulance Stroke Screen, Emergency Medical Stroke Assessment, etc. Among these scales, the FAST and BEFAST provide the highest diagnostic accuracy and are also linked to improved outcomes [Figure 1].[18,19]
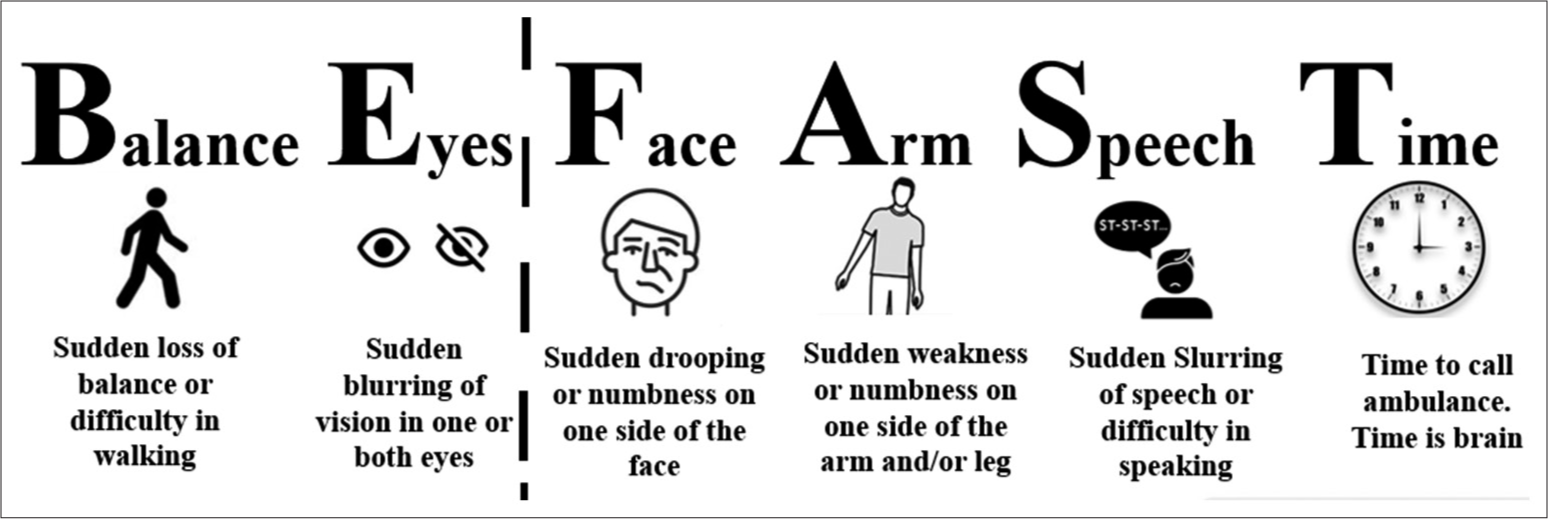
- BEFAST (balance, eyes, face, arm, speech, and time) and FAST (Face, arm, speech, and time) screening tools for rapid identification of acute stroke.
The golden hour workup for AIS
The primary goal of triage and the initial assessment following acute stroke is to stabilize the airway, breathing, and circulation. Following this, a brief and focused neurological assessment is warranted to determine whether the presenting features are consistent with acute stroke and, to know whether the patient is eligible for IVT with rtPA to treat AIS. To optimize the AIS workup, the National Institute of Neurological Disorders and Stroke has established time targets for critical actions during the golden hour [Table 2].[20] The time of symptom onset is defined as the time when the patient was “last seen normal” without the signs and symptoms of the current stroke. The use of a stroke severity rating scale, preferably, the NIHSS [Table 3],[17,21] is recommended (COR-I, LOE-BNR) to quantify the degree of neurological deficit, facilitate communication, help identify patients for fibrinolytic or mechanical intervention, allow objective measurement of changing clinical status, and identify those at higher risk for complications such as ICH.[17] All patients with suspected acute stroke should receive emergency brain imaging evaluation on their first arrival at a hospital before initiating any specific therapy to treat AIS (COR-I, LOE-A).[17] Noncontract computed tomography (NCCT, COR-I, and LOE-A) or magnetic resonance (MR, COR-I, and LOE-BNR) imaging are both effective in excluding ICH before IV alteplase administration.[17] However, MR imaging (MRI) is time-consuming and the precise sequences must be determined on a case-by-case basis. In patients who awake with stroke or have unclear time of onset >4.5 h from baseline or last known well, MRI to identify diffusion-positive fluid-attenuated inversion recovery–negative lesions can be useful for selecting those who can benefit from IV alteplase administration within 4.5 h of stroke symptom recognition (COR-IIa and LOE-B-R).[17]
| Clinical action | NINDS time target |
|---|---|
| Door-to-physician/provider evaluation | 10 min or less |
| Door-to-stroke team notification | 15 min or less |
| Door-to-completion of CT scan | 25 min or less |
| Door-to-interpretation of CT scan | 45 min or less |
| Door-to-treatment with fibrinolytic therapy | 60 min or less |
NINDS: National institute of neurological disorders and stroke, CT: Computed tomography, AIS: Acute ischemic stroke.
| 1a. Level of consciousness | 0=alert 1=drowsy 2=obtunded 3=coma/unresponsive |
| 1b. Level of consciousness questions (2) What is the month? What is your age? |
0=answers two questions correctly 1=answers one question correctly 2=answers neither question correctly |
| 1c. Level of consciousness commands (2) Open and close your eyes Grip and release your hand |
0=performs both tasks correctly 1=performs one task correctly 2=performs neither task correctly |
| 2. Best gaze | 0=normal horizontal movements 1=partial gaze palsy 2=complete gaze palsy |
| 3. Visual fields | 0=no visual field defect 1=partial hemianopia 2=complete hemianopia 3=bilateral hemianopia |
| 4. Facial movement | 0=normal symmetric movements 1=minor facial weakness 2=partial facial weakness 3=complete unilateral palsy |
| 5. Motor function (arm) 5a. Left arm 5b. Right arm |
0=no drift 1=drift before 10 s 2=falls before 10 s 3=no effort against gravity 4=no movement |
| 6. Motor function (leg) 6a. Left leg 6b. Right leg |
0=no drift 1=drift before 5 s 2=falls before 5 s 3=no effort against gravity 4=no movement |
| 7. Limb ataxia | 0=absent 1=present in one limb 2=present in two limbs |
| 8. Sensory | 0=normal, no sensory loss 1=mild-to-moderate sensory loss 2=severe to total sensory loss |
| 9. Best language | 0=no aphasia, normal 1=mild to moderate aphasia 2=severe aphasia 3=mute, global aphasia |
| 10. Dysarthria | 0=normal 1=mild-to-moderate dysarthria 2=severe dysarthria |
| 11. Extinction and inattention | 0=no abnormality 1=mild loss (1 sensory modality lost) 2=severe loss (2 modalities lost) |
Total Score: 0–42; Score 0: No stroke, Score 1–4: Mild stroke, Score 5–15: Moderate stroke, Score 15–20: Moderate to severe stroke, Score 21–42: Severe stroke
The benefit of IV alteplase therapy is time-dependent, and treatment should be initiated as quickly as possible (COR-I and LOE-A). Only the assessment of blood glucose must precede the initiation of IV alteplase in all patients (COR-I and LOE-B-NR).[17] Baseline electrocardiographic (COR-I and LOE-B-NR) and troponin (COR-I and LOE-C-LD) assessments are recommended in patients presenting with AIS but should not delay initiation of IV alteplase. The same holds true for international normalized ratio, activated partial thromboplastin time, and platelet count.[17]
IVT
IV alteplase is the first US Food and Drug Administration (FDA) approved drug for intravenous thrombolysis in AIS. Unless contraindicated [Table 4],[17] IV alteplase (0.9 mg/kg, maximum dose 90 mg over 60 min with initial 10% of dose given as bolus over 1 min) is recommended for selected patients who can be treated within 3 h of ischemic stroke symptom onset or patient last known well or at baseline state (COR-I and LOE-A).[17] However, the same regimen is also recommended for selected patients who can be treated within 3 and 4.5 h of ischemic stroke (COR-I and LOEB-R). These include patients ≤80 years of age, without a history of both diabetes mellitus and prior stroke, NIHSS score ≤25, not taking any oral anticoagulants, and without imaging evidence of ischemic injury involving more than one-third of the middle cerebral artery (MCA) territory.[17] Patients eligible for IV alteplase should receive it even if EVT is being considered (COR-I and LOE-A).[17] It is important to remember that for otherwise eligible patients with mild non-disabling stroke symptoms (NIHSS score 0–5), IV alteplase is not recommended for patients who could be treated within 3 h (COR III: No Benefit, LOE: B-R) or 4.5 h (COR III: No Benefit, LOE: C-LD) of ischemic stroke symptom onset or patient last known well or at baseline state.[17] These recommendations align with the European Stroke Organization guidelines on IVT for AIS.[22]
| Clinical |
|---|
| Mild non-disabling stroke (NIHSS score 0–5) |
| CT brain imaging exhibits extensive regions of clear hypoattenuation, defined as obvious hypodensity |
| CT brain imaging reveals intracranial hemorrhage |
| Acute head trauma |
| Ischemic stroke within the past 3 months |
| Severe head trauma within the past 3 months |
| Intracranial or intraspinal surgery within the past 3 months |
| History of intracranial hemorrhage |
| Symptoms and signs most consistent with an SAH |
| Structural GI malignancy or recent bleeding within the past 21 days |
| Coagulopathy defined as platelet count<100,000/mm3, INR >1.7, aPTT >40 s, or PT >15 s |
| Full treatment dose of low-molecular-weight within the past 24 h |
| Use of factor Xa inhibitors and direct thrombin inhibitors within the past 48 h |
| Symptoms consistent with infective endocarditis |
| Known or suspected to be associated with aortic arch dissection |
| Co-existing intracranial neoplasm |
IV: Intravenous, tPA: Tissue plasminogen activator, INR: International normalized ratio, aPTT: Activated partial thromboplastin time, NIHSS: National institutes of health stroke scale, SAH: Subarachnoid hemorrhage, AIS: Acute ischemic stroke, GI: Gastrointestinal.
Physicians should be prepared to treat potential emergent adverse effects during and after IVT, including bleeding complications and angioedema that may cause partial airway obstruction (COR I, LOE: B-NR).[17] Infusion should be promptly discontinued and a NCCT scan should be immediately obtained to exclude ICH if there is any new-onset severe headache, acute hypertension, nausea, vomiting, and/or worsening of neurologic status.[23] Patients should be admitted to an intensive care unit since continued hemodynamic and neurologic monitoring is needed for 24 h post-infusion. Standard post rt-PA blood pressure (BP) monitoring is recommended every 15 min for 2 h, every 30 min for 6 h, and hourly for the remainder of the 24 h after treatment with rt-PA.[17] Antiplatelet or anticoagulation therapy and invasive procedures should be withheld during the subsequent 24 h. An immediate neurosurgical consultation should be initiated during a post-rt-PA hemorrhage. Placement of nasogastric tubes, indwelling bladder catheters, or intra-arterial pressure catheters should preferably be delayed unless necessary.[17] The exact time frame is not defined; however, their placement should not delay the initiation of IV alteplase.
Tenecteplase, a genetically modified variant of alteplase, is gaining widespread use in the treatment of AIS and received FDA approval in March 2025. Intravenous tenecteplase in a single bolus dose of 0.25 mg/kg (maximum 25 mg) may be considered a reasonable alternative to IV alteplase for IVT (COR-IIb, LOE-B-R).[17] A recent meta-analysis that included 11 randomized controlled trials (RCTs) comprising a total of 3788 patients found a similar safety profile between tenecteplase 0.25 mg/kg and alteplase while showing that tenecteplase is superior to alteplase regarding excellent functional outcome and reduced disability at 3 months.[24] The results of this meta-analysis echo the findings of a few other meta-analyses published recently.[25-27] The pertinent advantages of tenecteplase over alteplase include single bolus administration due to its longer half-life (22 vs. 4 min for alteplase), 15-fold higher specificity for fibrin and an 80-fold decreased binding affinity to plasminogen activator inhibitor-1.[28]
EVT
EVT with or without IVT in large vessel occlusions (LVOs) can be considered within 0–6 h (the 1st h being golden hour) either with a stent retriever (COR-I and LOE-A) or direct aspiration (COR-I and LOE-B-R) technique if the patients meet the following criteria: [17]
Prestroke mRS score of 0–1
Causative occlusion of the internal carotid artery (ICA) or MCA segment 1 (M1)
Age ≥18 years
NIHSS score of ≥6
Alberta stroke program early computed tomography score (ASPECTS) of ≥6
Treatment can be initiated (groin puncture) within 6 h of symptom onset.
Further, the benefits of EVT using stent retrievers may also apply to patients who can begin treatment within 6 h of symptom onset and meet specific criteria. These criteria include a pre-stroke mRS score greater than 1, an ASPECTS score below 6, or an NIHSS score under 6, with a confirmed occlusion of the ICA or proximal MCA (M1) (COR-IIb and LOE-B-R) or anterior cerebral arteries, vertebral arteries, basilar artery, or posterior cerebral arteries (COR-IIb and LOE-C-LD).[17] The four recent trials (Rescue-Japan LIMIT Trial, ANGEL-ASPECT Trial, SELECT 2 Trial, and TENSION Trial) have shown that EVT with or without IVT even in patients with large ischemic strokes (ASPECTS Score 3–5, average core volume >50 mL) is associated with improved functional outcome, quality of life, and overall survival.[29-32] The discussion on EVT within the 6-–24-h therapeutic window is beyond the scope of this narrative update.
The technical objective of EVT should be to achieve reperfusion with an mTICI grade 2 b/3 angiographic result, optimizing the likelihood of a favorable functional clinical outcome (COR-I and LOE-A).[17]
BP management
During the hyperacute phase of AIS, it is crucial to continuously monitor BP since extremes of BP are linked to poor outcomes.[23] Hypotension and hypovolemia should be corrected to maintain systemic perfusion levels necessary to support organ function (COR-I and LOE-C-EO).[17] Hypertension is not uncommon in patients with AIS and is attributable to multiple factors, such as chronic hypertension, sympathetic surge, and disturbance in cerebrovascular autoregulation.[33] Control of high BP is warranted before performing IVT or EVT due to the possibility of a higher risk of ICH following the procedures. It is recommended to reduce BP ≤185/110 mm Hg in both IVT (COR-I and LOEB-R) and EVT (COR-IIa and LOE-B-NR) eligible patients before the procedure. Following IVT or EVT, BP should be maintained at <180/105 mm Hg for at least the first 24 h. A recent meta-analysis published in 2024 which included 4 major RCTs encompassing 1559 participants found that standard BP control (systolic BP ≤180 mm Hg) during the first 24 h post-EVT for AIS with LVOs was associated with better functional outcomes as compared to intensive BP control (systolic BP <140 mm Hg). In safety outcomes, there was no significant difference in all-cause mortality, any ICH, symptomatic ICH, parenchymal hematoma type 2, and stroke recurrence.[34] However, in patients with BP ≥220/120 mm Hg who do not receive IVT or EVT and have no comorbid conditions (concomitant acute coronary event, acute heart failure, aortic dissection, post fibrinolysis symptomatic intracranial hemorrhage, or preeclampsia/eclampsia) requiring urgent antihypertensive treatment, the benefit of initiating or reinitiating treatment of hypertension within the first 48–72 h is uncertain. It might be reasonable to lower BP by 15% during the first 24 h after onset of stroke (COR-IIb, LOE-C-EO). There is no clear evidence on the best pharmacologic agent for lowering BP during the hyperacute period of AIS. Different drug options (labetalol, nicardipine, clevidipine, hydralazine, etc.) may be appropriate depending on the expertise of the clinician and the availability of the drug [Table 5].[17]
| Patient otherwise eligible for emergency reperfusion therapy except that BP is >185/110 mm Hg: |
| Labetalol 10–20 mg IV over 1–2 min, may repeat 1 time; or |
| Nicardipine 5 mg/h IV, titrate up by 2.5 mg/h every 5–15 min, maximum 15 mg/h; when desired BP reached, adjust to maintain proper BP limits; or |
| Clevidipine 1–2 mg/h IV, titrate by doubling the dose every 2–5 min until desired BP reached; maximum 21 mg/h |
| Other agents (e.g., hydralazine and enalaprilat) may also be considered |
| If systolic BP>180–230 mm Hg or diastolic BP >105–120 mm Hg: |
| Labetalol 10 mg IV followed by continuous IV infusion 2–8 mg/min; or |
| Nicardipine 5 mg/h IV, titrate up to desired effect by 2.5 mg/h every 5–15 min, maximum 15 mg/h; or |
| Clevidipine 1–2 mg/h IV, titrate by doubling the dose every 2–5 min until desired BP reached; maximum 21 mg/h |
| If BP not controlled or diastolic BP>140 mm Hg, consider IV sodium nitroprusside |
BP: Blood pressure, IV: Intravenous.
Golden hour supportive management
Blood glucose
Evidence consistently suggests that both hypoglycemia and hyperglycemia are associated with poor outcomes after AIS. Liberal blood glucose level (140–180 mg/dL) is now recommended (COR-IIa and LOE-C-LD).[17]
Temperature
Both hypothermia and hyperthermia are linked to unfavorable outcomes following AIS. For that matter, induced hypothermia has not demonstrated any significant benefit in enhancing neurological outcomes after AIS (CORIIb and LOE-B-R). The cause of hyperthermia (temperature >38°C) should be promptly identified and addressed. In hyperthermic patients, antipyretic medications are recommended to reduce fever (COR-I, LOE- C-LD).[17]
Antimicrobial therapy
The routine administration of prophylactic antibiotics has demonstrated effectiveness in lowering infection rates but no improvement in functional outcomes; therefore, it is not recommended (COR-III and LOE-A).[17]
Anti-seizure therapy
Recurrent seizures following a stroke should be managed with anti-seizure medications based on the patient’s specific characteristics (COR-I and LOE-C-LD). However, the prophylactic use of anti-seizure drugs is not recommended (COR-III, no benefit; and LOE-C-LD).[17]
FUTURE DIRECTIONS – THE ROAD AHEAD
All hospitals managing stroke patients within a stroke care system should establish, implement, and follow care protocols aligned with the latest guidelines set by national and international professional organizations, as well as state and federal regulations (COR-I and LOE-C-EO). Engaging in a stroke data repository is advised to ensure consistent adherence to current treatment guidelines, support ongoing quality improvement, and enhance patient outcomes (COR-I, LOE-B-NR). The data repository can help identify gaps or disparities in the quality of stroke care. Once these gaps are recognized, targeted interventions can be implemented to address them (COR-I and LOE- B-NR).[17]
Artificial intelligence has a transformative role in improving diagnosis (computed tomography/MRI images analysis and perfusion imaging interpretation), workflow automation (automated alerts, triage and routing, and integrated communication), treatment (IVT and EVT suitability, and dose optimization), and outcomes (prognostic models) during the critical “golden hour” following an AIS. However, the clinical application of these technologies still faces challenges such as limitations in data volume, model interpretability, and the need for real-time monitoring and updating. Future progress will depend on multidisciplinary collaboration, the development of interpretable models, the establishment of comprehensive imaging databases, and continuous algorithm refinement. The potential of large language models, such as those based on the transformer architecture in stroke imaging analysis, opens up new research avenues, promising more personalized and effective stroke management strategies.[35-37]
CONCLUSION
The golden hour is vital in acute ischemic stroke care, offering the best chance to reduce brain damage and improve outcomes. Rapid recognition, emergency response, imaging, and timely thrombolysis and/or thrombectomy are essential during this window. While stroke care systems and early intervention protocols have advanced, challenges like delayed access and low public awareness remain. Strengthening education, streamlining hospital processes, and promoting coordinated care are key to overcoming these barriers. Reinforcing the importance of the golden hour can significantly enhance survival, reduce disability, and improve the overall effectiveness of stroke management.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required, as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Global, regional, and national burden of stroke and its risk factors 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795-820.
- [CrossRef] [PubMed] [Google Scholar]
- Global, regional, and national burden of stroke and its risk factors 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23:973-1003.
- [Google Scholar]
- Pragmatic solutions to reduce the global burden of stroke: A World Stroke Organization-Lancet Neurology Commission. Lancet Neurol. 2023;22:1160-206.
- [CrossRef] [PubMed] [Google Scholar]
- The burden, risk factors and unique etiologies of stroke in South-East Asia Region (SEAR) Lancet Reg Health Southeast Asia. 2023;17:100290.
- [CrossRef] [PubMed] [Google Scholar]
- Diverging temporal trends in stroke incidence in younger vs older people: A systematic review and meta-analysis. JAMA Neurol. 2022;79:1036-48.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-29.
- [CrossRef] [PubMed] [Google Scholar]
- Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929-35.
- [CrossRef] [PubMed] [Google Scholar]
- Golden hour treatment with tPA (Tissue-Type Plasminogen Activator) in the BEST-MSU study. Stroke. 2023;54:415-25.
- [CrossRef] [PubMed] [Google Scholar]
- IV tPA given in the golden hour for emergent large vessel occlusion stroke improves recanalization rates and clinical outcomes. J Neurol Sci. 2021;428:117580.
- [CrossRef] [PubMed] [Google Scholar]
- The golden hour of acute ischemic stroke. Scand J Trauma Resusc Emerg Med. 2017;25:54.
- [CrossRef] [PubMed] [Google Scholar]
- Intravenous thrombolysis for ischemic stroke in the golden hour: Propensity-matched analysis from the SITS-EAST registry. J Neurol. 2017;264:912-20.
- [CrossRef] [PubMed] [Google Scholar]
- Golden Hour intravenous thrombolysis for acute ischemic stroke: A systematic review and meta-analysis. Ann Neurol. 2024;96:582-90.
- [CrossRef] [PubMed] [Google Scholar]
- Golden Hour thrombolysis in acute ischemic stroke: The changing pattern in South Korea. J Stroke. 2021;23:135-8.
- [CrossRef] [PubMed] [Google Scholar]
- The “golden hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41:1431-9.
- [CrossRef] [PubMed] [Google Scholar]
- Academic emergency medicine in India. Emerg Med Australas. 2013;25:359-64.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344-418.
- [CrossRef] [Google Scholar]
- A Systematic review and meta-analysis comparing FAST and BEFAST in acute stroke patients. Front Neurol. 2022;12:765069.
- [CrossRef] [PubMed] [Google Scholar]
- Mnemonic utilization in stroke education: FAST and BEFAST adoption by certified comprehensive stroke centers. Front Neurol. 2024;15:1359131.
- [CrossRef] [PubMed] [Google Scholar]
- The golden hour performing an acute ischemic stroke workup. Nurs Pract. 2014;39:22-9. quiz 29-30
- [CrossRef] [PubMed] [Google Scholar]
- NIH Stroke Scale reliability in ratings from a large sample of clinicians. Cerebrovasc Dis. 2006;22:389-95.
- [CrossRef] [PubMed] [Google Scholar]
- European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I-LXII.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
- [CrossRef] [PubMed] [Google Scholar]
- Tenecteplase vs alteplase in acute ischemic stroke within 4.5 hours: A systematic review and meta-analysis of randomized trials. Neurology. 2024;103:e209903.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety outcomes of Tenecteplase versus Alteplase for thrombolysis of acute ischemic stroke: A meta-analysis of 9 randomized controlled trials. J Neurol Sci. 2024;458:122912.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of tenecteplase versus alteplase for acute ischemic stroke: An updated systematic review, pairwise, and network meta-analysis of randomized controlled trials. J Thromb Thrombolysis. 2023;55:322-38.
- [CrossRef] [PubMed] [Google Scholar]
- Tenecteplase versus alteplase for patients with acute ischemic stroke: A meta-analysis of randomized controlled trials. Aging (Albany NY). 2023;15:14889-99.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41:1229-45.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386:1303-13.
- [CrossRef] [PubMed] [Google Scholar]
- Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023;388:1272-83.
- [CrossRef] [PubMed] [Google Scholar]
- Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023;388:1259-71.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular thrombectomy for acute ischaemic stroke with established large infarct: Multicentre, open-label, randomised trial. Lancet. 2023;402:1753-63.
- [CrossRef] [PubMed] [Google Scholar]
- Acute hypertensive response in patients with stroke: Pathophysiology and management. Circulation. 2008;118:176-87.
- [CrossRef] [PubMed] [Google Scholar]
- Standard versus intensive blood pressure control in acute ischemic stroke patients successfully treated with endovascular thrombectomy: A systemic review and meta-analysis of randomized controlled trials. J Stroke. 2024;26:54-63.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence and acute stroke imaging. AJNR Am J Neuroradiol. 2021;42:2-11.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence in ischemic stroke images: Current applications and future directions. Front Neurol. 2024;15:1418060.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence applied in acute ischemic stroke: From child to elderly. Radiol Med. 2024;129:83-92.
- [CrossRef] [PubMed] [Google Scholar]







