Translate this page into:
Efficacy and safety of dual-antiplatelet therapy with high-intensity statin versus single-antiplatelet therapy with high-intensity statin in patients with stroke or high-risk mini-stroke of atherosclerotic origin: A cohort study
*Corresponding author: Asawari Raut, PhD. Department of Pharmacy Practice, Bharati Vidyapeeth (Deemed to be University) Poona College of Pharmacy, Pune, Maharashtra, India. asawari.raut@bharatividyapeeth.edu
-
Received: ,
Accepted: ,
How to cite this article: Suryawanshi VR, Patwal S, Doshi V, Iyer S, Raut A. Efficacy and safety of dual-antiplatelet therapy with high-intensity statin versus single-antiplatelet therapy with high-intensity statin in patients with stroke or high-risk mini-stroke of atherosclerotic origin: A cohort study. J Neurosci Rural Pract. 2025;16:44-54. doi: 10.25259/JNRP_278_2024
Abstract
Objectives:
The aim was to study the efficacy and safety of two treatment regimes for stroke or high-risk transient ischemic attack (TIA) of atherosclerotic origin.
Materials and Methods:
A prospective observational cohort study was conducted in patients with stroke (National Institutes of Health Stroke Scale [NIHSS], 1–10; atherosclerotic-origin) who did not undergo thrombolysis or thrombectomy and in patients with high-risk TIA (ABCD2, ≥4). Two treatment regimes studied included; dual-antiplatelet therapy (DAPT) (1–21 days) followed by single-antiplatelet therapy (SAPT) (22–90 days) with high-intensity statin (HIS) for 90 days (Group-A) versus SAPT for 90 days with HIS for 60 days (Group-B). Patients were followed up for efficacy endpoints including prevention of early neurological deterioration (END), new stroke/TIA, and neurofunctional recovery at three months. The safety endpoints included a composite of cardiovascular events, bleeding events, and muscle-toxic effects. A multivariate logistic regression and Cox-proportional hazards model were used to evaluate endpoints.
Results:
Of 160 patients, 82 completed Group-A therapy, and 78 completed Group-B therapy. The NIHSS for qualifying stroke was median (Interquartile range) 5 (2–8). END occurred in 2.4% (Group-A) versus 7.7% (Group-B) patients (hazards ratio [HR], 0.66; 95% confidence interval [CI], 0.42–0.91; P = 0.08). A new stroke/TIA occurred in 4.8% (Group-A) versus 11.5% (Group-B) patients (HR, 0.75; 95% CI, 0.61–0.93; P = 0.06). A change in the severity of stroke or high-risk TIA combined with a modified Rankin scale toward favorable outcomes was observed in Group A (odds ratio, 3.12; 95% CI, 1.71–5.52; P = 0.001). Though the risk was minimal in both cohorts, bleeding events and muscle-toxic effects were 4.7 and 4.6% points higher in Group-A patients.
Conclusion:
Compared to Group-B therapy, Group-A therapy was found to be more effective in preventing END and new stroke/TIA, and in improving neurofunctional recovery at three months, albeit at the expense of minimal safety hazards. Multicentric and randomized controlled trials are required for generalization of the study findings.
Keywords
Atherosclerosis
Dual antiplatelet
High-intensity statin
High-risk transient ischemic attack
Stroke
INTRODUCTION
Two separate but prevalent subtypes of stroke of atherosclerotic origin in the Asian population are intracranial atherosclerotic disease (ICAD), often referred to as intracranial steno-occlusive disease, and branch atheromatous disease (BAD).[1,2] A rise in the National Institutes of Health Stroke Scale (NIHSS) score of ≥2 in 7 days relative to baseline is considered early neurological deterioration (END).[3] According to reports, END progresses quickly, with an incidence of 20–40% in strokes caused by ICAD or BAD.[4] Stroke or high-risk transient ischemic attack (TIA, also referred to as “mini-stroke”) patients have about 15–20% likelihood of developing yet another stroke within three months from its first occurrence.[5] Patients with minor stroke (defined as NIHSS of ≤3 on a scale of 0–42, with higher scores implying severe stroke) who can receive treatment within 24 h of symptom onset are advised to receive dual-antiplatelet therapy (DAPT) consisting of clopidogrel and aspirin, according to evidence-based guidelines.[6] Many stroke patients have a lower chance of receiving DAPT due to this timeframe and low NIHSS score criterion. According to data from the pooled analysis of the CHANCE and POINT trials,[7-9] DAPT (given for 21 days) could aid patients with high-risk TIA or minor strokes by reducing the probability of experiencing a prolonged ischemic event if it is administered for a maximum of 72 h after the stroke onset. These studies featured all types of non-embolic strokes. Furthermore, a few studies have revealed that DAPT can reduce the potential for END.[8-10] From the findings of a network meta-analysis in patients with large atherosclerotic strokes, DAPT was found to be beneficial in preventing recurrent stroke, myocardial infarction, and all-cause mortality.[11] Considering the most recent evidence (published at the time of writing this study report), the ATAMIS trial evaluated the effect of DAPT (for 1–14 days) followed by single-antiplatelet therapy (SAPT) (for 15–90 days) versus SAPT (for 90 days) in adults with mild-to-moderate stroke (NIHSS, 4–10) and admitted within 48 h of symptom onset.[12] The results from the trial suggest that DAPT was superior in preventing END at 7 days compared to SAPT with a risk difference of −1.9% (−3.6% to −0.2%, P = 0.03) with a similar safety profile.[12] In patients with symptomatic ICAD, DAPT appeared to be more effective than SAPT at preventing secondary strokes without raising the risk of bleeding, according to published research. However, its impact on BAD-related stroke remains still unclear.
Regardless of their baseline low-density lipoprotein cholesterol levels, the guideline suggests patients with stroke or high-risk TIA must started on moderate-to-high intensity statin therapy as soon as possible (primarily within 72 h) to lower the risk of recurrent stroke and composite of cardiovascular events.[6] A high-resolution magnetic resonance imaging (MRI) study demonstrates that high-intensity statin (HIS) therapy effectively stabilizes symbolic intracranial atherosclerotic plaques throughout the acute period of stroke and high-risk TIA, in addition to improving neurofunctional outcomes and reducing NIHSS scores.[4] Having said this, the utilization of statin therapy in patients with stroke still remains inadequate (the exception may be atherosclerotic stroke), despite its widespread use in cardiovascular diseases.[13] Studies support the use of statins in patients who have experienced a stroke, as they reduce the risk of recurrent stroke and do not increase the risk of hemorrhagic stroke.
Our hospital is compliant with American heart association (AHA)/American stroke association (ASA) guideline-based recommendations (quality metrics), which were already documented in our previous study,[14] where we observed particularly good complaint rates associated with the use of early antithrombotics and statins. However, treatment outcomes associated with the use of DAPT with HIS in ICAD/BAD-associated stroke or high-risk TIA have never been studied systematically in our setting. A similar paucity was also observed considering the countrywide scenario, exception is one study from the comprehensive stroke care program in Kerala.[15] For atherosclerotic diseases (ICAD or BAD), antiplatelets and statins are the two important treatment modalities. However, only a few studies described the three-month clinical outcomes associated with its use in this population, especially in Indian patients.[15] As ICAD and BAD exhibit relatively similar pathogenesis,[2] we hypothesize that early intensive medical therapy with short-term DAPT combined with HIS for 90 days could prevent END and recurrent stroke/TIA within three months in a larger number of patients compared to SAPT combined with HIS for 60 days. We, therefore, investigate the same in this study.
MATERIALS AND METHODS
Primary research question?
Whether the patients with stroke or high-risk TIA of atherosclerotic origin could benefit from DAPT followed by SAPT combined with HIS (for 90 days) in terms of preventing END and recurrent stroke compared to only SAPT combined with HIS (for 60 days)? What is the distribution of neurofunctional outcome scores on a modified Rankin scale (mRS) at three months?
Study design, patients, and oversight
We conducted a prospective-observational cohort analysis of patients who arrived from urban-suburban regions and got admitted with atheromatous disease-related stroke or high-risk TIA. The study site was a tertiary-care university hospital situated in Pune, India. We enrolled all the patients with stroke and high-risk TIA aged ≥18 years. The study was conducted for a period of one year and six months. The study was approved by the Institutional Ethics Committee of the study hospital (Reference Number: BVDUMC/IEC/2022/224). The patients received a study information sheet, and informed consent was obtained before their enrollment. The study was conducted in compliance with the international committee on harmonization of good clinical practice (ICH-GCP) guidelines adopted in the 18th World Medical Assembly, which apply to clinical research carried out in India.[16]
Study enrollment was for patients who had either experienced a high-risk TIA with a score of ≥4 on the ABCD2 scale (which measures the risk of stroke based on patient demographics, vitals like blood pressure, TIA duration, clinical features, and current status of diabetes mellitus; score - 0–7, with higher scores implying greater stroke risk) or minor-to-moderate stroke with a NIHSS score of 1–10. Other important eligibility criteria were as follows: (a) Patients who received DAPT or SAPT with HIS within 24–48 h of last known stroke/TIA symptom onset; (b) pre-stroke (baseline) mRS score of ≤5; (c) suspected or diagnosed of having ICAD/BAD with an ischemic lesion on diffuse-weighted imaging (DWI) located in striatocapsular territory or brain stem areas, with an axial diameter of ≤20 mm; (d) intracranial arterial stenosis was defined as presence of 50–99% stenosis (Warfarin-Aspirin Symptomatic Intracranial Disease trial criteria)[17] in major intracranial arteries including internal carotid artery (ICA), anterior cerebral artery, posterior cerebral artery, middle cerebral artery (MCA), thalamic artery, and basilar artery, determined by magnetic resonance angiography; (e) patients with BAD, either suspected or confirmed (detectable ischemic lesion in three or more axial cuts on DWI in the lenticulostriate area, or as infarcts extending from the basal surface of the pons); and (f) ability to tolerate high-intensity drug therapy, including 75–300 mg/day of aspirin, 300 mg of loading clopidogrel, 75–150 mg after day 2, and HISs (rosuvastatin 20–40 mg/day or atorvastatin 40–80 mg/day).
The following patients were excluded: (a) Those who necessitated endarterectomy, endovascular intervention, or immediate thrombolysis; (b) those who had received intravenous or intra-arterial thrombolysis less than a week before the index event; (c) patients with aortic dissection, cervicocerebral artery dissection, vasculitis, vascular malformation, moyamoya disease, fibromuscular dysplasia, or cardioembolic stroke has been identified as the cause of their stroke or TIA; (d) patients with clear indications for anticoagulation (deep vein thrombosis, pulmonary embolism, or hypercoagulable state) during the study period; (e) contraindications to clopidogrel, aspirin, atorvastatin, or rosuvastatin; and (f) anticipated need for long-term (>7 days) non-study antiplatelet/antithrombotic drugs (e.g., dipyridamole, ticagrelor, and ticlopidine) were excluded from the study.
Study cohorts and data abstraction
Patients who received DAPT or SAPT in combination with HIS as per stroke and high-risk TIA management and prevention guidelines were categorized,[18] and based on the treatment duration for DAPT or SAPT and HIS, they were divided into two cohorts, which are defined as: (a) Group-A: Patients who received DAPT for 1–21 days followed by SAPT for 22–90 days with HIS for 90 days; (b) Group-B: Patients who received SAPT for 90 days with HIS for 60 days. All the patients received standard care as recommended in the AHA/ASA’s latest guidelines in addition to DAPT/SAPT and HIS.[6,18] DAPT consisted of clopidogrel 300 mg plus aspirin 325 mg on day 1; clopidogrel 75 mg plus aspirin 75 mg on days 2–21. SAPT consisted of aspirin 75–150 mg on days 1–14; and aspirin 75 mg on days 15–90. The patients with stroke or high-risk TIA were treated by the clinical judgment of the treating physician (considering real-world clinical practice). The decisions behind administering either group therapy were based on stroke severity, radio-imaging findings, small artery or large artery atherosclerotic disease, lacunar infarcts, risks of hemorrhage, intracranial or extracranial or both diseases and other obvious risk factors.[6,18,19] The patients were initiated on either group therapies in an emergency department or intensive-care units.
Medical history and history of presenting illness were obtained from the patients (if their level of consciousness and/or neurological deficits precluded it) and/or caregivers. Patient medical case files and concern reports were reviewed, and the required data were abstracted in a pre-designed patient profile form. The patient data that were extracted are as follows: (a) Baseline characteristics and demographics, vascular risk factors, and comorbid conditions; (b) clinical condition on emergency medicine department (EMD) arrival (including level of consciousness assessed using the Glasgow coma scale and stroke severity assessed using the NIHSS); (c) the presence of acute infarcts on DWI or CT, as well as the location, degree of stenosis, and vascular territory, were assessed separately on baseline brain and vascular imaging, blinded to outcome data; and (d) therapeutic interventions provided, and the data regarding clinical outcomes.
Study endpoints
The study patients were followed up for a period of three months. Outcome assessment was performed in medicine and neurology OPDs at the end of three months. Whereas for the patients who did not show up in OPDs, a separate telephonic conversation and video meeting were arranged to understand the neurofunctional recovery at the end of two months and three months.
The primary endpoint measure was the percentage of patients with END, which was defined as an increase in NIHSS by ≥2 within seven days compared to baseline NIHSS. Another primary endpoint was the development of a new stroke (ischemic or hemorrhagic) or TIA at three months. The secondary endpoint measurements comprised a composite of poor functional outcome (mRS, 3–6) and cardiovascular events (myocardial infarction, stroke, or death from cardiovascular causes) that occurred within three months. Percentage of patients who had a favorable neurofunctional outcome (FNO) defined by mRS score of ≤2 at three months. The score “Barthel index (BI score, 90–100) for activities of daily living” was also recognized as a FNO.
Major safety endpoints for HIS therapy included hepatotoxic events (aspartate or alanine aminotransferase levels ≥5 times of normal) and muscle-toxic effects (creatine phosphokinase levels ≥10 times of normal with or without myopathy, rhabdomyolysis, or myalgias). Bleeding events attributed to DAPT/SAPT were another safety endpoint, where mild-to-moderate bleeding was defined as mucocutaneous or gastrointestinal or related bleeding, and severe bleeding was defined as intracerebral or subarachnoid or subdural hemorrhage. These definitions were in accord with “The Heidelberg Bleeding Classification” of bleeding events after stroke and reperfusion therapies.[20] All clinical outcomes, comprising recurrent stroke, myocardial infarction, mortality, and bleeding events, were evaluated by an impartial clinical event adjudication committee before final decisions were made. Figure 1 represents patient selection and study flow.
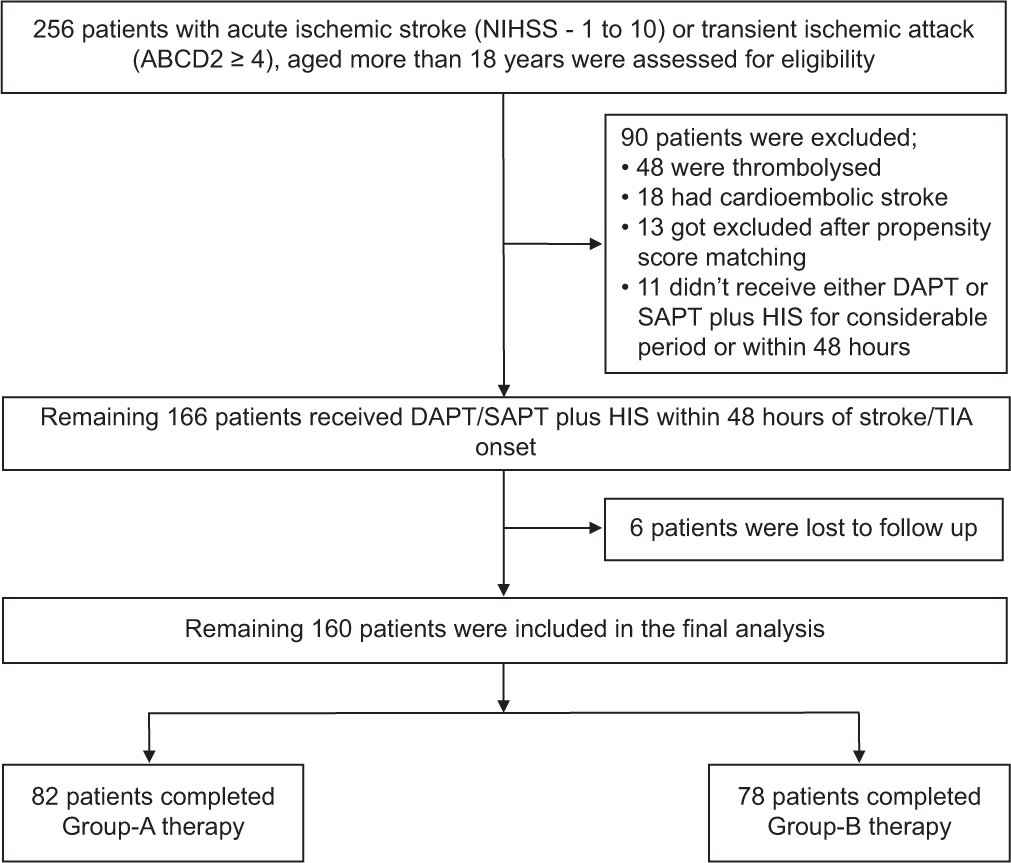
- Patient selection and study flow. Description: The final analysis did not include patients who were lost to follow-up, had been enrolled improperly, or had stopped receiving study group therapies because they were intolerable or had other problems of that nature. The national institute of health stroke scale has a score range of 0–42; higher values indicate significant neurologic impairments. Increased scores on the ABCD2 scale (from 0 to 7) indicate increased risk of having stroke. NIHSS: National institutes of health stroke scale, DAPT: Dual-antiplatelet therapy, SAPT: Single-antiplatelet therapy, HIS: High-intensity statin, TIA: Transient ischemic attack.
Statistical analysis
An END has been demonstrated to occur typically in patients with ICAD or BAD following a single subcortical infarction, with a frequency ranging from 27% to 40% in different studies.[10,21] Patients with BAD who received DAPT may have a reduction in the END rate to 9.7%.[22] Based on prior trials, we estimated that the aspirin group would have an 11.5% risk of new stroke/TIA during a three-month period, whereas DAPT would result in 18–20% reduced risk.[8-10] With a sample size of 73 in each cohort, the study would require 90% power to detect a hazard ratio of 0.8 in favor of Group A at a two-sided alpha level of 0.05. Five percentage losses to follow-up and screening failure rates were allowed for a total sample size of 158. The mean (standard deviation [SD]) or median (interquartile range [IQR]) were utilized to represent the nominal variables, while proportions were utilized to describe the categorical variables. Wherever necessary and appropriate, the difference between the two groups was evaluated using an Analysis of variance or Mann– Whitney’s U-test for continuous variables, and the χ2 test was used to examine categorical data (for non-continuous variables). Predefined confounders were measured and balanced between the two groups using the propensity score matching analysis. Independent determinants of the measured outcomes were examined using a logistic regression model. Variables from the univariate analysis with P < 0.1 were included in the multivariate logistic analysis using forward selection techniques. The differences between Group A and Group B in terms of the risk of END and new stroke/TIA at three months were assessed using the marginal Cox proportional-hazards model after patient characteristics were taken into consideration. Comparable techniques were used to compare the secondary endpoints. To evaluate the likelihood of muscle-toxic effects, hepato-toxic effects, bleeding events, and a poor neurofunctional result, a generalized linear model and relative risks (RRs) with 95% CIs were used. All statistical analyses were performed using Microsoft Excel version 16.80 and the Statistical Package for the Social Sciences version 26.0 software. Each author was accountable for preserving the integrity of the data and had unrestricted access to it.
RESULTS
Patient demographics, clinical characteristics, and time windows
Of the total 160 patients (100 males and 60 females) diagnosed with either stroke or TIA and who provided consent for enrollment in the study, 82 (51.2%) patients received Group-A therapy, and the remaining 78 (48.8%) patients received Group-B therapy. The patient characteristics of stroke and high-risk TIA admissions did not differ in both groups at baseline [Table 1]. Overall, the mean (SD) age of study participants was 59.2 (12.1) years. The most striking risk factors included hypertension (63.1%), followed by diabetes mellitus (49.4%), dyslipidemia and coronary artery disease (22.5%), and previous or current smoking (20.6%). Other important risk factors included carotid artery stenosis (15.6%), tobacco use (10.6%), and previous stroke (9%). Most (91%) of the patients had their first-ever stroke or TIA. Most of the patients (65%) had acute multiple infarctions (AMIs) on radioimaging (ipsilateral or contralateral to the cerebrum), followed by acute single infarction (ASIs) (23%), and TIA (12%). At admission, the NIHSS score in qualifying stroke patients was median (IQR) 5 (2–8). The majority (54.4%) of patients had minor strokes (score ≤4), followed by mild-to-moderate stroke (score 5–10; 44.6%). Similarly, considering the ABCD2 score in qualifying TIA, 37% of patients were at high risk for developing stroke (ABCD2, 6–7), and the remaining 63% of patients were at moderate risk for developing stroke (ABCD2, 4–5). Seventy-eight (48.7%) patients had MCA involvement as the affected vascular territory, followed by 40 (25%) patients with ICA involvement. The majority of 112 (70%) patients received atorvastatin 80 mg, followed by 44 (27.5%) receiving atorvastatin 40 mg, and 4 (2.5%) receiving rosuvastatin 20 mg. The median (IQR) time of symptom onset to hospitalization was 10.5 (5–22) h. The time of onset of stroke symptoms to administration of antiplatelets was 13.4 (7.7–35.3) h. The median (IQR) length of hospitalization was 7 (4–10) days.
| Variables | Total (N=160), n(%) | Group A (n=82), n(%) | Group B (n=78), n(%) | P-value* |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 59.2 (12.1) | 58.6 (12) | 59.8 (12.3) | NS |
| Median (IQR) | 60 (50-69) | 60 (50-67) | 61 (53-71) | |
| Gender | ||||
| Male | 100 (62) | 54 (65.8) | 46 (58.9) | NS |
| Female | 60 (38) | 28 (34.2) | 32 (41.1) | NS |
| BMI (kg/m2) | ||||
| Mean (SD) | 24.3 (3.4) | 23.8 (3.3) | 24.8 (3.5) | NS |
| Stroke risk factors | ||||
| Hypertension | 101 (63.1) | 47 (57.3) | 54 (69.2) | 0.094 |
| Diabetes mellitus | 79 (49.4) | 35 (42.7) | 44 (56.4) | 0.081 |
| Dyslipidemia and CAD | 36 (22.5) | 16 (19.5) | 20 (25.6) | NS |
| Previous or current smoking | 33 (20.6) | 16 (19.5) | 17 (21.8) | NS |
| Carotid artery stenosis | 25 (15.6) | 10 (12.2) | 15 (19.2) | NS |
| Tobacco use | 17 (10.6) | 9 (11) | 8 (10.3) | NS |
| Previous ischemic stroke | 14 (9) | 6 (7.3) | 8 (10.3) | NS |
| Qualifying event | ||||
| TIA | 19 (12) | 10 (12.1) | 9 (11.5) | NS |
| Acute single infarction | 37 (23) | 18 (21.9) | 19 (24.3) | NS |
| Acute multiple infarctions | 104 (65) | 54 (66) | 50 (64.2) | NS |
| NIHSS score in qualifying IS | ||||
| Median (IQR) | 5 (2–8) | 5 (2–8) | 5 (1–8) | NS |
| Score≤4 | 78 (55.4) | 42 (58.3) | 36 (52.2) | NS |
| Score 5–10 | 63 (44.6) | 30 (41.7) | 33 (47.8) | NS |
| ABCD2score in qualifying TIA | ||||
| 4–5 | 12 (63) | 7 (70) | 5 (55) | NS |
| >5 | 7 (37) | 3 (30) | 4 (45) | NS |
| Vascular territory | ||||
| Middle cerebral artery | 78 (48.7) | 44 (53.6) | 34 (43.6) | NS |
| Internal carotid artery | 40 (25) | 20 (24.4) | 20 (25.6) | NS |
| Posterior cerebral artery | 36 (22.5) | 16 (19.5) | 20 (25.6) | NS |
| Basilar artery | 28 (17.5) | 16 (19.5) | 12 (15.4) | NS |
| Thalamic artery | 20 (12.5) | 12 (14.6) | 8 (10.3) | NS |
| Anterior cerebral artery | 18 (11.2) | 8 (9.7) | 10 (12.8) | NS |
| Combination artery (internal carotid artery and middle cerebral artery) | 10 (6.2) | 7 (8.5) | 3 (3.8) | NS |
| Statins used | ||||
| Atorvastatin 80 mg | 112 (70) | 60 (73.2) | 52 (66.6) | NS |
| Atorvastatin 40 mg | 44 (27.5) | 18 (22) | 26 (33.3) | NS |
| Rosuvastatin 20 mg | 4 (2.5) | 4 (4.8) | 0 | NS |
| Time windows*, median(P25–P75) | ||||
| Symptom onset to EMD arrival | 10.5 (5–22) | 12 (5–20) | 10 (5–22.5) | NS |
| Door-to-imaging | 43 (30–70) | 42 (31–68) | 43 (29–70) | NS |
| Symptom onset to antiplatelet administration | 13.4 (7.7–35.3) | 14.2 (7.2–34.5) | 12.8 (7.3–36.5) | NS |
| Antiplatelet administration | ||||
| Within 12 h | 60 (37.5) | 29 (35.4) | 31 (39.7) | NS |
| Within 13–24 h | 55 (34.3) | 29 (35.4) | 26 (33.3) | NS |
| Within 25–48 h | 45 (28.1) | 24 (29.2) | 21 (27) | NS |
| Length of stay (days) | ||||
| Mean (SD) | 7 (3.5) | 7 (3.5) | 7 (4.0) | NS |
| Median (IQR) | 7 (4–10) | 7 (4–10) | 7 (5–10) | NS |
SD: Standard deviation, IQR: Interquartile range, CAD: Coronary artery disease, CT: Computed tomography, MRI: Magnetic resonance imaging, EMD: Emergency medical department, NIHSS: National institute of health stroke scale, IS: Ischemic stroke, TIA: Transient ischemic attack, NS: Not significant. *Eligible patients only (patients with missing data were excluded from this analyses). The propensity score matching analysis was used to measure and balance predetermined covariates between the two groups
Efficacy and safety outcomes
An END (primary efficacy outcome) was observed in 2 patients (2.4%) in Group A and 6 patients (7.7%) in Group B (marginal estimated hazards ratio [HR], 0.66; 95% confidence interval [CI], 0.42–0.91; P = 0.08). A new stroke/TIA (recurrence) occurred in 4 patients (4.8%) from Group A and 9 patients (11.5%) from Group B (HR, 0.75; 95% CI, 0.61–0.93; P = 0.06). The absolute risk reduction was 7% (95% CI, 3.67–12.67), which was in favor of Group A for new stroke/TIA within three months.
With regard to secondary outcomes, a composite of cardiovascular events occurred in 4 patients (4.8%) in Group A and 7 patients (8.9%) in Group B (HR, 0.80; 95% CI, 0.65–1.02; P = 0.12). Considering the distribution of mRS score, poor neurofunctional outcome (mRS, 3–6) has occurred in 24 patients (29%) in Group A and 44 patients (56%) in Group B (RR, 0.35; 95% CI [0.21–0.48]; P = 0.001). A change in the severity of stroke and high-risk TIA at three months combined with the mRS score toward a FNO was observed in Group-A patients compared to Group-B (odds ratio [OR], 3.12; 95% CI, 1.71–5.52; P = 0.001). Similarly, using the BI score for favorable functional recovery (score, 90–100), Group-A patients had significant recovery compared to Group-B (OR, 3.61; 95% CI, 2.11–5.48; P = 0.001).
There were no significant differences in hepatotoxic effects. Considering primary safety outcomes, muscle-toxic effects occurred in 8 (9.7%) patients in Group A and 4 (5.1%) patients in Group B (RR, 1.66; 95% CI, 0.85–2.77; P = 0.15). Bleeding events occurred in 7 (8.5%) patients in Group A and 3 patients (3.8%) in Group B (RR, 1.93; 95% CI, 0.33–2.84; P= 0.13). All the patients (Group-A [n = 6] plus Group-B [n = 3]) had mild-to-moderate bleeding events related to antiplatelet use, whereas one patient from Group A had a severe bleeding event in the form of subarachnoid hemorrhage, which prolonged the hospitalization and postponed the neurofunctional recovery. The patients with muscle-toxic effects and bleeding events led to discontinuation or non-adherence to either arm therapies, resulting in poor neurofunctional outcomes at three months. Other efficacy and safety endpoints are represented in Table 2.
| Variables | Group-A (n=82), n (%) | Group-B (n=78), n (%) | Hazards ratio or relative risk (95% CI) | P-value |
|---|---|---|---|---|
| END* | 2 (2.4) | 6 (7.7) | 0.66 (0.42–0.91) | 0.081 |
| New stroke/TIA at 3 months | 4 (4.8) | 9 (11.5) | 0.75 (0.61–0.93) | 0.062 |
| Composite cardiovascular event | 4 (4.8) | 7 (8.9) | 0.80 (0.65–1.02) | 0.124 |
| (stroke, MI, or death from cardiovascular cause) | ||||
| Myocardial infarction | 2 (2.4) | 4 (5.1) | 0.55 (0.24–1.01) | 0.183 |
| Death from cardiovascular cause | 2 (2.4) | 5 (6.4) | 0.39 (0.18–0.77) | 0.122 |
| Poor neurofunctional outcome (mRS 3–6)† | 24 (29) | 44 (56) | 0.35 (0.21–0.48) | 0.001 |
| Severe grade neurofunctional deficits (BI 0–20) | 12 (14.6) | 20 (25.6) | 0.54 (0.27–0.89) | 0.054 |
| Mild-to-moderate grade neurofunctional deficits (BI 20–90) | 16 (19.6) | 31 (39.7) | 0.32 (0.22–0.59) | 0.007 |
| Favorable neurofunctional outcome (mRS≤2)# | 58 (71) | 34 (44) | 3.12 (1.71–5.52) | 0.001 |
| Favorable neurofunctional recovery (BI 90–100)# | 54 (65.8) | 27 (34.7) | 3.61 (2.11–5.48) | 0.001 |
| Rehospitalization (with or without stroke) | 4 (4.8) | 8 (10.3) | 0.47 (0.17–1.16) | 0.123 |
| Hepatotoxic effects† | 4 (4.8) | 3 (3.8) | 1.05 (0.57–1.65) | 0.212 |
| Muscle toxic effects† | 8 (9.7) | 4 (5.1) | 1.66 (0.85–2.77) | 0.157 |
| Bleeding-events† | 7 (8.5) | 3 (3.8) | 1.93 (0.33–2.84) | 0.134 |
DAPT: Dual-antiplatelet therapy, SAPT: Single-antiplatelet therapy, HIS: High-intensity statin, mRS: Modified Rankin scale, BI: Barthel index, END: Early neurological deterioration, CI: Confidence interval. *END is defined as an increase of NIHSS of ≥2 at day 7 compared to baseline NIHSS or recurrent stroke/TIA within 30 days. #Adjusted odds are shown for favorable neurofunctional outcome and recovery. †Relative risks are shown for poor neurofunctional outcomes, and safety outcomes
In the subgroup analysis with age >50 years, 65.5% of Group-A patients and 38.7% of Group-B patients had FNO (OR, 2.91; 95% CI, 1.05–3.27; P < 0.001). With regard to AMIs, Group-A patients (64.8%) had FNO compared to Group-B patients (30%) (OR, 3.25; 95% CI, 2.11–4.17; P < 0.001). Considering an NIHSS score of 5–10, 60.4% and 41.8% of patients from Groups A and B, respectively, had FNO (OR, 2.07; 95% CI, 1.18–3.64; P= 0.011). In the subgroup of ≥ 60% symptomatic stenosis, Group-A patients (66.6%) depicted significantly higher FNO compared to Group-B patients (45.2%) (OR, 2.16; 95% CI, 1.26–3.51; P < 0.001). Patients who received Group-A therapy within 12, 24, and 48 h of the index event had higher odds of FNO, compared to patients who received Group-B therapy. MCA and ICA were found to be the most common vascular territories of infarctions, wherein patients who received Group-A therapy compared to Group-B therapy had higher odds of FNO at the end of three months (OR = 3.25 [MCA territory] and 2.56 [ICA territory], P < 0.001) [Table 3].
| Subgroup | Group-A (n=82) | Group-B (n=78) | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|
| No. of patients with favorable outcome/Total no. (%) | No. of patients with favorable outcome/Total no. (%) | |||
| Age | ||||
| ≤50 y | 20/24 (83.3) | 10/16 (62.5) | 2.86 (1.48–3.84) | <0.001 |
| >50 y | 38/58 (65.5) | 24/62 (38.7) | 2.91 (1.05–3.27) | <0.001 |
| Gender | ||||
| Male | 36/54 (66.6) | 21/46 (45.6) | 2.48 (1.12–3.41) | 0.002 |
| Female | 22/28 (78.5) | 13/32 (40.6) | 3.54 (1.73–5.21) | 0.003 |
| Qualifying event | ||||
| TIA | 9/10 (90) | 7/9 (77.7) | 2.53 (1.33–4.56) | 0.021 |
| ASI | 14/18 (77.7) | 9/19 (47.3) | 3.08 (2.11–4.88) | 0.001 |
| AMIs | 35/54 (64.8) | 18/50 (30) | 3.25 (2.11–4.17) | <0.001 |
| NIHSS score in qualifying stroke | ||||
| Score 1–4 | 32/39 (82.1) | 16/35 (45.7) | 3.82 (2.21–5.54) | <0.001 |
| Score 5–10 | 26/43 (60.4) | 18/43 (41.8) | 2.07 (1.18–3.64) | 0.011 |
| LDL-C at Baseline | ||||
| ≤100 mg/dL | 28/34 (82.1) | 18/33 (54.5) | 3.81 (1.81–4.13) | <0.001 |
| >100 mg/dL | 30/48 (62.5) | 16/45 (35.5) | 3.65 (1.24–3.71) | <0.001 |
| ≥60% symptomatic stenosis | ||||
| Yes | 30/45 (66.6) | 19/42 (45.2) | 2.16 (1.26–3.51) | <0.001 |
| No | 28/37 (75.6) | 15/36 (41.6) | 3.72 (2.13–4.54) | <0.001 |
| Time of antiplatelet administration | ||||
| Within 12 h | 23/29 (79.3) | 16/31 (51.6) | 3.58 (1.15–8.28) | 0.025 |
| Within 13–24 h | 20/29 (68.9) | 11/26 (42.3) | 3.03 (1.10–7.15) | 0.047 |
| Within 25–48 h | 15/24 (62.5) | 7/21 (33.3) | 3.33 (0.98–9.35) | 0.051 |
| Vascular territory of infarction | ||||
| MCA | 33/44 (75) | 15/34 (44.1) | 3.25 (2.14–4.68) | <0.001 |
| ICA | 13/20 (65) | 8/20 (40) | 2.56 (1.53–3.51) | <0.001 |
| PCA | 8/16 (80) | 12/20 (46.1) | 3.63 (2.41–4.81) | <0.001 |
| BA | 12/16 (75) | 8/12 (66.6) | 1.21 (0.81–2.23) | 0.108 |
| TA | 6/12 (50) | 2/8 (25) | 3.24 (2.15–5.68) | <0.001 |
| ACA | 5/8 (62.5) | 6/10 (60) | 1.05 (0.55–1.42) | 0.321 |
| Combination artery (ICA and MCA) | 5/7 (71.4) | 1/3 (33.3) | 4.83 (2.74–6.42) | <0.001 |
TIA: Transient ischemic attack, ASI: Acute single infarction, AMIs: Acute multiple infarctions, NIHSS: National institute of health stroke scale, OR: Odds ratio, LDL: Low-density lipoprotein, CCA: Common carotid artery, MCA: Middle cerebral artery, ICA: Internal carotid artery, ACA: Anterior cerebral artery, PCA: Posterior cerebral artery, TA: Thalamic artery, BA: Basilar artery, CI: Confidence interval
The mean (SD) mRS in the Group-A cohort was 3 (1.5) at admission and 2 (1.1) at the end of three months. In a similar way, it was 3 (1.6) and 3 (0.8) in the Group-B study cohort, respectively. When the Group-A cohort’s and Group-B cohort’s neurofunctional outcomes were examined, statistically significant differences were observed (P < 0.01) ([Figure 2a] Temporal changes in mRS). Similar statistically significant differences in neurofunctional recovery were also observed when comparing the mean (SD) BI scores between the study cohorts (Group-A vs. Group-B) (89 [10] vs. 66 [15], P < 0.01) [Figure 2b].
![(a) Temporal changes in modified Rankin scale (mRS) assessed on admission and at three months. Description: In Group-A study cohort, the mean (Standard deviation [SD]) mRS at admission and at three months was 3 (1.5) and 2 (1.1), respectively. Similarly, in Group-B study cohort, it was 3 (1.6) and 3 (0.8), respectively. Comparing neurofunctional outcome (Group-A vs. Group-B), statistically significant differences were observed with P < 0.01. (b) Neurofunctional outcomes assessed using the Barthel index at three months. Description: Comparing the mean (SD) Barthel index (BI) score between the study cohorts (Group-A vs. Group-B), statistically significant differences were observed in neurofunctional recovery (89 [10] vs. 66 [15], P < 0.01).](/content/150/2025/16/1/img/JNRP-16-044-g002.png)
- (a) Temporal changes in modified Rankin scale (mRS) assessed on admission and at three months. Description: In Group-A study cohort, the mean (Standard deviation [SD]) mRS at admission and at three months was 3 (1.5) and 2 (1.1), respectively. Similarly, in Group-B study cohort, it was 3 (1.6) and 3 (0.8), respectively. Comparing neurofunctional outcome (Group-A vs. Group-B), statistically significant differences were observed with P < 0.01. (b) Neurofunctional outcomes assessed using the Barthel index at three months. Description: Comparing the mean (SD) Barthel index (BI) score between the study cohorts (Group-A vs. Group-B), statistically significant differences were observed in neurofunctional recovery (89 [10] vs. 66 [15], P < 0.01).
DISCUSSION
In this prospective-observational cohort study, the patients with atherosclerotic stroke or high-risk TIA and who received Group-A therapy within 48 h of the onset of stroke symptoms had a risk of END and new stroke/TIA at three months that was roughly 5.3 and 6.7% points lower than the risk of END and new stroke/TIA with Group-B therapy. While this risk was minimal in both study cohort participants, Group-A patients had a roughly 4.7 and 4.6%-point higher risk of bleeding events and muscle-toxic effects, respectively, than Group-B patients. The demographics and clinical characteristics of the patients in our study were well compared to those in earlier studies.[9,10,12,14,15]
The results from CHANCE, SAMMPRIS, and POINT trials demonstrate the persistent risk of recurrent stroke or TIA (5.3–7.6% at 30 days, 10.1–11.3% at three months, and 12.5% at 1 year) despite continued use of antiplatelet and statins.[8,10,23-25] Having said this, the subgroup analysis from the present study demonstrated that the patients whose stroke incidence is linked to major artery atherosclerosis and who received long-term DAPT and HIS benefited most in terms of secondary prevention and END. For secondary prevention and better neurofunctional sequela, now there is recent randomized controlled trial (RCT) evidence to support the use of HIS in combination with DAPT. The trial suggests that the therapy can be taken up to 72 h after stroke or high-risk TIA onset.[9] Our analysis showed the patients who received DAPT and HIS within 24–48 h of stroke or high-risk TIA onset depicted adequate prevention of END and new stroke/TIA within three months, alongside patients who also exhibited significant neurofunctional recovery at three months follow-up.
The CHANCE trial has sparked a few worries as the antiplatelets administered after the trial’s conclusion may confound the results of the trial’s treatments. Considering its well-established efficacy in preventing secondary strokes, antiplatelets administered after 90 days are anticipated to have a beneficial effect on stroke recurrence prevention within three months to one year.[8] DAPT was associated with a 3.5% decreased incidence of new stroke/TIA in the CHANCE study compared to aspirin alone (SAPT) when therapy was initiated within 24 h.[8] This difference in percentage points was higher than what was shown in the INSPIRES trial (2%-points, HR, 0.79; 95% CI, 0.66–0.94; P = 0.008).[9] The longer randomization period in the ISPIRES trial compared to the CHANCE trial, the fact that only roughly 13% of the patients in the INSPIRES trial received treatment within 24 h, and the higher risk of stroke recurrence during the acute post-stroke phase could all contribute to these differences, whereas almost 50% of the patients in CHANCE trial got randomized to treatment arm within 12 h. Hence, “time is brain” in treating stroke patients. Our analysis also supports this finding with a percentage-point difference of 6.7. About 72% of patients in our study received either group therapy within 24 h (median, 13.4 h) of stroke symptom onset. Comparing aspirin (SAPT) to DAPT, the findings from the INSPIRES trial correspond to an approximate number of 54 that must be treated to avoid one more stroke (absolute risk reduction, 1.87%; 95% CI, 0.49–3.25%). Whereas in our analysis, the method resulted in a 6.7% point decrease in the new stroke/TIA within three months (absolute risk reduction, 7%; 95% CI, 1.67–14.27%), corresponding to an approximate number of 15 patients that must be treated with Group-A therapy to avoid one more stroke that would have happened under Group-B therapy. Our study found that the incidence of overall bleeding events was 8.5% for Group A and 3.8% for Group B. These rates are higher than the risk that most previous studies have reported (2–4% associated with DAPT) and more than twice as high as the aspirin-alone risk (1–2%) that was reported in the previous series of studies,[8-10,21,23,26] the potential reason behind this could be a small sample size in our study.
In accordance with trials conducted in ICAD/BAD patients, the percutaneous transluminal angioplasty and stenting result in increased risks of new stroke/TIA and un FNOs compared to aggressive medical therapy, including DAPT/SAPT and moderate-to-HIS.[24,25] Hence, HIS remains an important therapy in these patients, and it needs to be combined with antiplatelets. When comparing low-to-moderate intensity or no statin therapy to patients with stroke, whether they had atrial fibrillation or not, there was a decreased risk of net adverse clinical and cerebral events and an improved likelihood of favorable outcomes. This was especially true for 75 years of age or older patients and patients who had undergone revascularization therapy.[27] Our results signified that HIS therapy for 90 days (Group-A) provides a better prognosis and improved neurofunctional recovery compared to HIS for 30–60 days (Group B) in all subgroups of patients (especially patients aged >50 years, NIHSS score of 5–10, low-density lipoprotein cholesterol [LDL-C] >100 mg/dL, and >60% symptomatic stenosis in the affecting arteries). It is essential to take into consideration an array of variables while evaluating the benefits of HIS continued for 90 days in patients with ICAD/BAD-related stroke. Similar results have also been reported by STAMINA-MRI and other studies.[4,13,28] Important benefits of HIS include a reduction in the risk of increasing cerebral infarction volume and prevention of END and recurrent stroke, which may be primarily achieved due to the stabilization of atherosclerotic plaques and a decrease in LDL-C levels.[28,29] Studies demonstrate that after three months, individuals treated with dose-time-responsive statins have better neurofunctional outcomes and considerably greater survival rates over a three years period. In light of this, the findings point out the importance of statins’ pleiotropic effects (neuroprotective, antioxidant, collateral circulation-promoting, and anti-inflammatory effects), which could benefit stroke patients in the long run by promoting neurofunctional recovery, long-term survival, and preventing recurrent vascular events.[30,31]
Our findings indicate that, overall, for every 1000 patients treated with Group-A therapy for acute stroke (NIHSS ≤10) and high-risk TIA (ABCD2 ≥4), there would be essentially 53 fewer ENDs, 67 fewer new strokes/TIAs, and 10 more bleeding-events anticipated compared with Group-B therapy. A few limitations considering the exclusion of certain significant patient groups with stroke or TIA from this study, including the patients admitted with moderate-to-severe stroke (NIHSS, >10) and the patients who had undergone mechanical thrombectomy or thrombolysis (in study hospital or outside study hospital). Another clinically relevant factor could be clopidogrel resistance. The CYP3A4 and CYP2C19 genotype variants and P2Y12 gene polymorphisms have a substantial influence on the prognosis of patients with stroke and their responsiveness to clopidogrel.[32,33] Therefore, the outcomes of our investigations may have limited generalizability.
Study highlights
DAPT followed by SAPT combined with HIS for 90 days could benefit stroke patients with age 35–65 years, NIHSS 5–10, degree of stenosis >60%, acute single-to-multiple infarctions, and ICA-MCA vascular territory involvement.
Administration of DAPT for 1–21 days followed by SAPT for 22–90 days with HIS for 90 days (initiated within 48 h of stroke/high-risk TIA symptom onset) was associated with higher odds of END prevention, recurrent stroke prevention, and neurofunctional recovery.
CONCLUSION
The results of this prospective-observational cohort study imply that the therapy from the Group-A cohort initiated within 24-48 hours after stroke symptom onset resulted in a decreased risk of END, averted new stroke/TIA, and improved the odds of neurofunctional recovery, albeit the effect comes with a minor risk of bleeding events and muscle-toxic effects compared to therapy from the Group-B cohort over a period of three months. Similar objectives should be studied using multicentric, randomized, and well-controlled trials to help clinicians generalize study findings effectively.
Ethical approval
The research/study approved by the Institutional Review Board at Bharati Vidyapeeth Deemed University Medical College, Pune-43., number BVDUMC/IEC/2022/224, dated December 01, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Ischemic stroke subtype classification: An Asian viewpoint. J Stroke. 2014;16:8-17.
- [CrossRef] [PubMed] [Google Scholar]
- Branch atheromatous disease: A clinically meaningful, yet unproven concept. Cerebrovasc Dis. 2016;41:87-95.
- [CrossRef] [PubMed] [Google Scholar]
- Early neurological deterioration (END) after stroke: The END depends on the definition. Int J Stroke. 2011;6:211-2.
- [CrossRef] [PubMed] [Google Scholar]
- Intensive statin treatment in acute ischaemic stroke patients with intracranial atherosclerosis: A high-resolution magnetic resonance imaging study (STAMINA-MRI Study) J Neurol Neurosurg Psychiatry. 2020;91:204-11.
- [CrossRef] [PubMed] [Google Scholar]
- One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-42.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344-418.
- [CrossRef] [Google Scholar]
- Outcomes associated with clopidogrel-aspirin use in minor stroke or transient ischemic attack: A pooled analysis of clopidogrel in high-risk patients with acute non-disabling cerebrovascular events (CHANCE) and platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trials. JAMA Neurol. 2019;76:1466-73.
- [CrossRef] [PubMed] [Google Scholar]
- Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: One-year outcomes. Circulation. 2015;132:40-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dual antiplatelet treatment up to 72 hours after ischemic stroke. N Engl J Med. 2023;389:2413-24.
- [CrossRef] [PubMed] [Google Scholar]
- Dual antiplatelet therapy in stroke and ICAS: Subgroup analysis of CHANCE. Neurology. 2015;85:1154-62.
- [CrossRef] [PubMed] [Google Scholar]
- Dual anti-platelet therapy for secondary prevention in intracranial atherosclerotic disease: A network meta-analysis. Ther Adv Neurol Disord. 2022;15:17562864221114716.
- [CrossRef] [PubMed] [Google Scholar]
- Clopidogrel plus aspirin vs aspirin alone in patients with acute mild to moderate stroke: The ATAMIS randomized clinical trial. JAMA Neurol. 2024;81:450-60.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of high-dose statins in acute phase of ischemic stroke and transient ischemic attack: A systematic review. Intern Emerg Med. 2017;12:679-87.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of quality metrics of acute stroke care with clinical outcomes in an Indian Tertiary-care university hospital: A prospective evidence-based study. Indian J Crit Care Med. 2023;27:806-15.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of risk factors, treatment, and outcome in patients with symptomatic intracranial atherosclerotic disease in India and the United States. Ann Indian Acad Neurol. 2020;23:265-9.
- [CrossRef] [PubMed] [Google Scholar]
- Good clinical practices for clinical research in India. Available from: https://drugscontrol.org/good-clinical-practice-guidelines.php [Last accessed on 2024 Jul 04]
- [Google Scholar]
- Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-16.
- [CrossRef] [PubMed] [Google Scholar]
- 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-467.
- [CrossRef] [Google Scholar]
- Acute ischemic stroke: Management approach. Indian J Crit Care Med. 2019;23(Suppl 2):S140-6.
- [CrossRef] [PubMed] [Google Scholar]
- The Heidelberg bleeding classification: Classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clopidogrel plus aspirin prevents early neurologic deterioration and improves 6-month outcome in patients with acute large artery atherosclerosis stroke. Clin Appl ThrombHemost. 2015;21:453-61.
- [CrossRef] [PubMed] [Google Scholar]
- Ultra-early combination antiplatelet therapy with cilostazol for the prevention of branch atheromatous disease: A multicenter prospective study. Cerebrovasc Dis Extra. 2016;6:84-95.
- [CrossRef] [PubMed] [Google Scholar]
- Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-25.
- [CrossRef] [PubMed] [Google Scholar]
- Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
- [CrossRef] [PubMed] [Google Scholar]
- Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): The final results of a randomised trial. Lancet. 2014;383:333-41.
- [CrossRef] [PubMed] [Google Scholar]
- Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of statin therapy on outcomes of patients with acute ischemic stroke and atrial fibrillation. J Am Heart Assoc. 2019;8:e013941.
- [CrossRef] [PubMed] [Google Scholar]
- High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-59.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of statins on atherosclerotic plaque. Trends Cardiovasc Med. 2019;29:451-5.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453-63.
- [CrossRef] [PubMed] [Google Scholar]
- Statins: Multiple mechanisms of action in the ischemic brain. Neuroscientist. 2007;13:208-13.
- [CrossRef] [PubMed] [Google Scholar]
- Clopidogrel resistance in patients with stroke recurrence under single or dual antiplatelet treatment. Front Neurol. 2021;12:652416.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of CYP2C19 and P2Y12 gene polymorphisms on clinical results of patients using clopidogrel after acute ischemic cerebrovascular disease. Balkan J Med Genet. 2015;17:37-41.
- [CrossRef] [PubMed] [Google Scholar]







