Translate this page into:
Differences in merlin and p53 expression as a predisposing factor in orbital meningioma
*Corresponding author: Raudatul Janah, Department of Anatomical Pathology, Cicendo Eye Hospital, Bandung, Indonesia. raudatul.janah1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Janah R, Rujito L, Wahyono DJ. Differences in merlin and p53 expression as a predisposing factor orbital meningioma. J Neurosci Rural Pract. 2024;15:477-83. doi: 10.25259/JNRP_642_2023
Abstract
Objectives:
The behavior of orbital meningiomas is difficult to predict. The p53 tumor suppressor gene mutation and the neurofibromatosis 2 gene’s inactivation in the merlin formation are two of the several mechanisms that contribute to the development of tumors. This considers the comparison of merlin and p53 expression as an inclination to evaluate the orbital meningiomas.
Materials and Methods:
This investigation is an observational expository considered within the shape of cross-sectional (cross-sectional). The samples/objects of this study were 44 patients with orbital meningioma who had a clinical, radiological, and histopathological diagnosis at the anatomical pathology laboratory at Cicendo Eye Hospital and Hasan Sadikin Bandung in 2017–2020, then an immunohistochemical examination of merlin and p53 expression was performed.
Results:
The study indicated that there was no relationship between p53 expression and orbital meningioma grading, also there is no relationship between merlin expression and orbital meningioma grading. However, based on the analysis test results, grade 3 orbital meningiomas tended to have a positive p53 expression rather than a negative expression and tend to have a negative merlin expression instead of a positive.
Conclusion:
Meningiomas with negative merlin expression have a tendency to express positive p53. Likewise, the higher grade (grade 3) tends to express positive p53 and negative merlin, which may play a key role in tumorigenesis of orbital meningioma, hence, an added value for clinical information and behavioral descriptions of orbital meningioma itself.
Keywords
Meningioma
Merlin
p53
INTRODUCTION
Meningiomas are common tumors of the central nervous system originating from the meningeal membranes of the brain and spinal cord.[1,2] Orbital meningiomas consist of primary optic nerve (ON) sheath meningiomas, primary intraorbital ectopic meningiomas, and spheno-orbital meningiomas based on their anatomical location.[3] While proptosis is typically present in secondary orbital meningioma (intracranial sphenoid wing meningioma) due to the tumor mass’s effect of compressing space, primary ON sheath meningioma typically causes loss of vision without pain.[4] The development of meningiomas is known to be caused by somatic mutations of the neurofibromatosis 2 (NF2) gene (monosomy and deletion of chromosome 22q),[5] and physiologically, NF2 produces the protein merlin. Merlin functions to activate cytoskeleton membrane proteins[6] and serves as a tumor suppressor in the proliferation and uncontrolled growth of arachnoid cells through inhibition of CUL4A-RBX1-DDB1-VprBD/DCAF1 E3 ubiquitin-protein ligase complex.[7,8] Mutation of the NF2 gene is the cause of the pathogenesis of sporadic meningiomas. Inactive NF2 causes merlin to not be produced. This can be caused by intrinsic NF2 mutations or from exogenous exposure such as chronic trauma to exposure to the steroid hormone (progesterone). This raises the possible role of steroid hormones in the development of meningioma.[9-11]
The behavior of meningiomas is difficult to predict because many factors are involved in tumor development, one of which is the such as tumor suppressor gene p53. p53 is a gene that encodes p53 through a tumor suppressor mechanism. The mechanism is identified when p53 gene mutation regulates cell cycle development, DNA repair, and apoptosis to function as a tumor suppressor gene.[12,13] The role of p53 gene mutations in meningioma tumorigenesis is controversial, and the trend in the literature suggests that p53 inactivation is associated with meningioma development.[14-17] The p53 gene mutation produces abnormal protein metabolites in the cell nucleus, where the expression level can be detected by immunohistochemical examination.[13,18] The P53 protein expression in cells is an indicator of p53 gene mutation. The P53 immunopositive and p53 mutations are associated with prognostic markers of meningioma development, but it still remained unclear whether these markers are expressed and associated with meningioma grading.[13] Several proposed hypotheses are involved in complex molecular pathways, such as the first p53 pathway in development. Meningiomas may be associated with NF2 inactivation and importantly through NF2 loss of heterozygosity, the second possible product of the NF2 protein, merlin, was reported to increase p53 stability through murine double minute 2 (Mdm2) downregulation in mouse fibroblasts.[12] To the best of our knowledge, there is no single evidence that examines the direct relationship of p53 expression, merlin, and orbital meningioma grading, so this study aims to determine the differences in the immunohistochemical expression of merlin and p53 with orbital meningioma grading because the markers of p53 and merlin expression are important in providing clinical information for patients.
MATERIALS AND METHODS
This study is a cross-sectional observational analysis, of 44 cases of histologically diagnosed orbital meningiomas in the anatomical pathology laboratory of Hasan Sadikin Hospital Bandung in 2017–2020. Formalin-fixed paraffin-embedded tissue blocks of meningiomas were reviewed by two pathologists and the World Health Organization (WHO) grades as defined by the 2016 WHO classification for meningiomas were assigned according to their histological features. The exclusion criteria are incomplete medical record data, tumor mass paraffin block is depleted, damaged. Immunohistochemical examination with paraffin-embedded tissue was obtained from the anatomical pathology laboratory at Hasan Sadikin Hospital in Bandung and Cicendo Eye Hospital in Bandung. The tissues were sliced 4 m with a microtome, deparaffinized, antigen-retrieved, endogenousperoxidase-blocked, and applied blocking solutions. We used monoclonal mouse anti-p53 (clone D07, cat No. BZ-088331FAM, Merck Inc., Darmstadt, Germany; 1:300 dilution), and anti-NF2 (cat No. BZ-0864950F-AP, Merck Inc., Darmstadt, Germany; 1:100 dilution). Samples were labeled using labeled streptavidin-biotin immunoperoxidase complex by One Step Neopoly Detection Kit (Biogear Scientific, BioVentures, Inc., Iowa, USA), visualized using 3,3’-diaminobenzidine, and counterstained using Harris hematoxylin. After dehydration, the samples were mounted and analyzed. The evaluation of immunoexpression from both biomarkers was analyzed using the Allred score. The Allred score combines the rate of positive cells and the concentration of the response item. The two scores were included for the last score with eight conceivable values, namely, S scores 0 and 2 are considered negative, while S scores of 3–8 are considered positive. Statistical analysis of p53 and merlin expression with orbital meningioma grading using the Mann–Whitney test with P < 0.05 is considered as significant. They were analyzed with SPSS for Windows version 24 software.
RESULTS
The results of the Mann–Whitney test are shown in Table 1. The significance value shows the number 0.095 or P > 0.05, indicative of. Due to P > 0.05, statistically, there was no relationship between P53 expression and orbital meningioma grading. Further, the Mann–Whitney test compared the rankings between the p53-positive expression groups having a higher rank than the negative p53-expression groups (23.4 vs. 13.5). Because the codes for grades 1, 2, and 3 are 0, for grade 2 is 1, and for grade 3 is 2, which means the higher grade of orbital meningioma increases with the code. A higher rating indicates a higher possible grade of orbital meningioma. Therefore, thus, what is meant by the relationship here is that grade 3 orbital meningiomas tend to have a positive p53 expression compared to negative expression. The p53 and merlin staining is shown in Figures 1-6.
| Ekspresi p53 (n, %) | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Nilai (P-value) |
|---|---|---|---|---|
| Negatif | 4 (9.1) | 0 (0) | 0 (0) | 0.095 |
| Positif | 22 (50) | 11 (25) | 7 (15.9) | |
| Total | 26 (59.1) | 11 (25) | 7 (15.9) |
Mann–Whitney test P<0.05, the mean p53 expression was negative 13.5%, p53 expression positive 23.4%
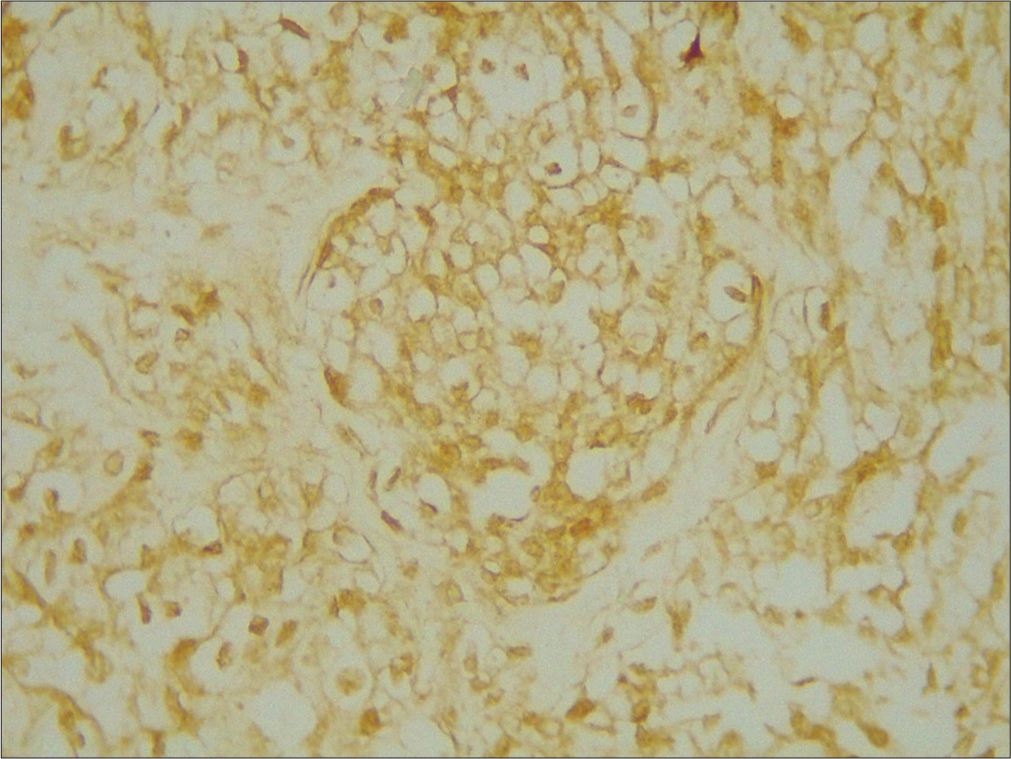
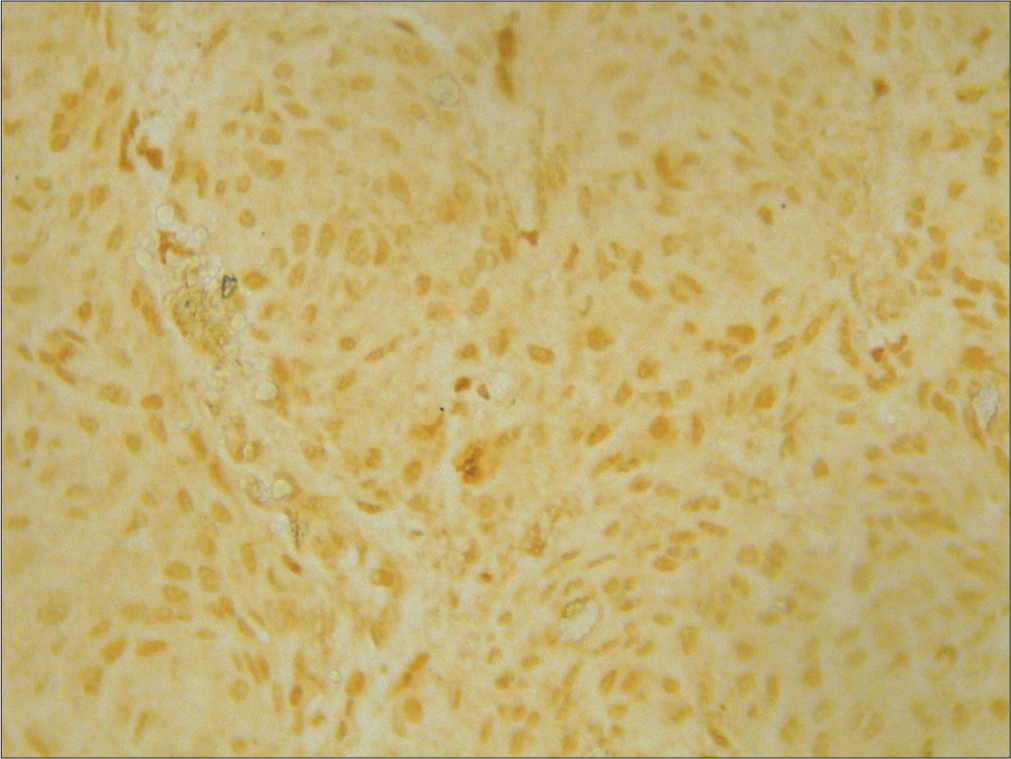
- ×400 merlin intermediate positive grade 2.
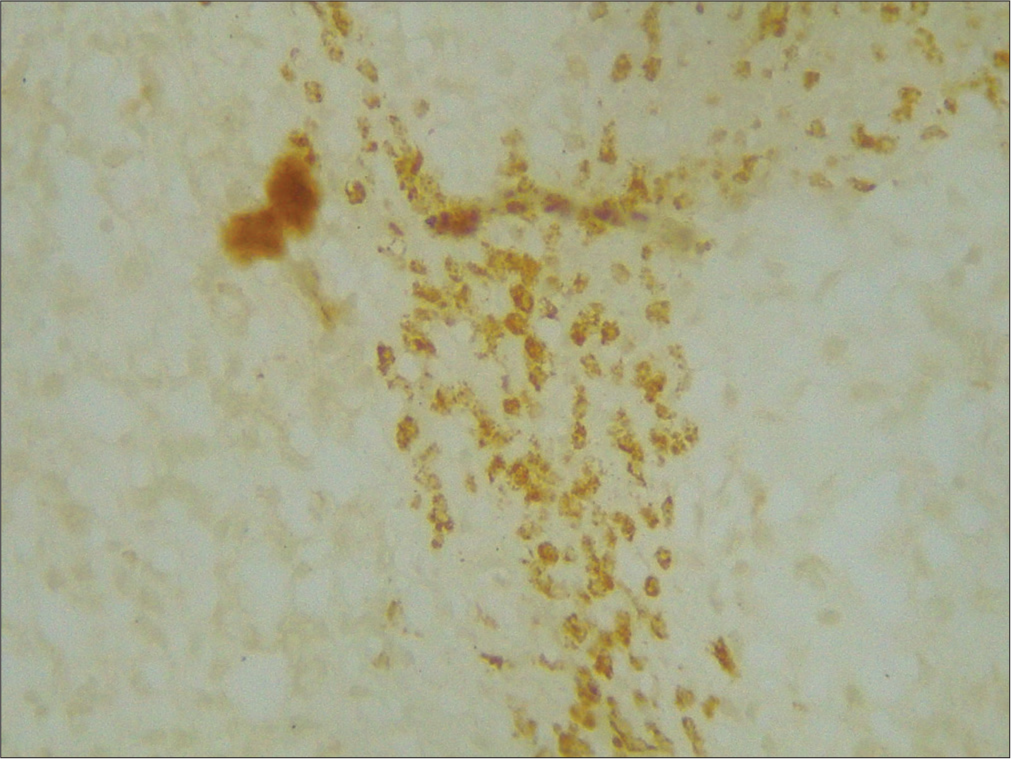
- ×400 merlin weak positive grade 3.
- ×400 merlin strong positive grade 1.
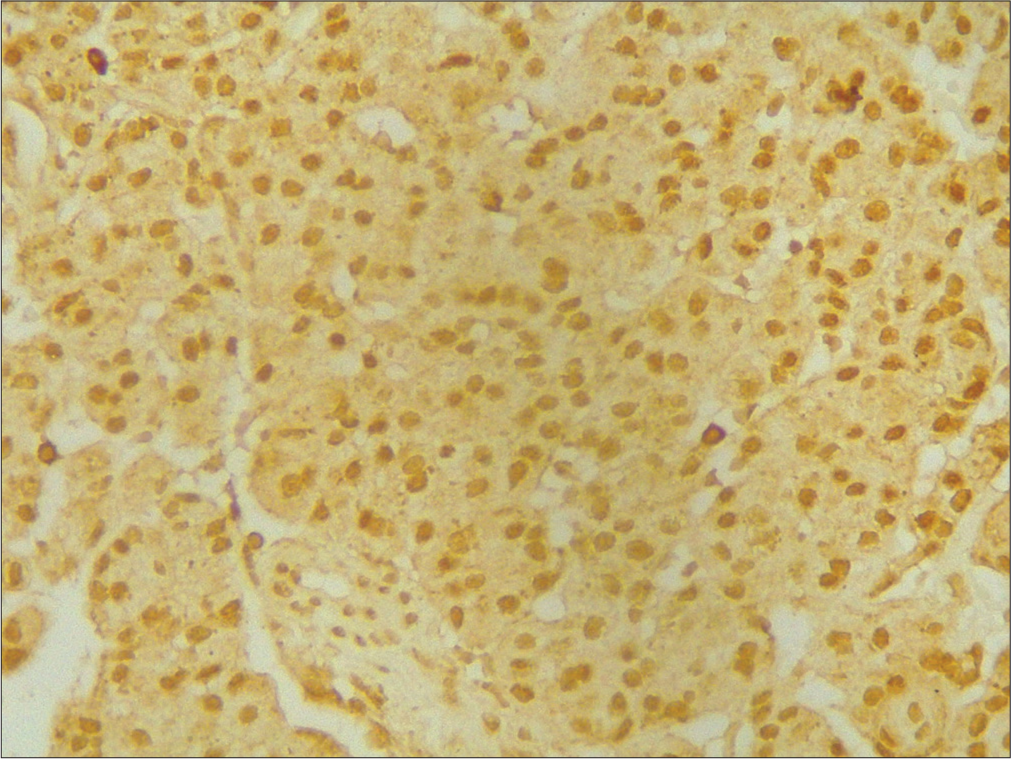
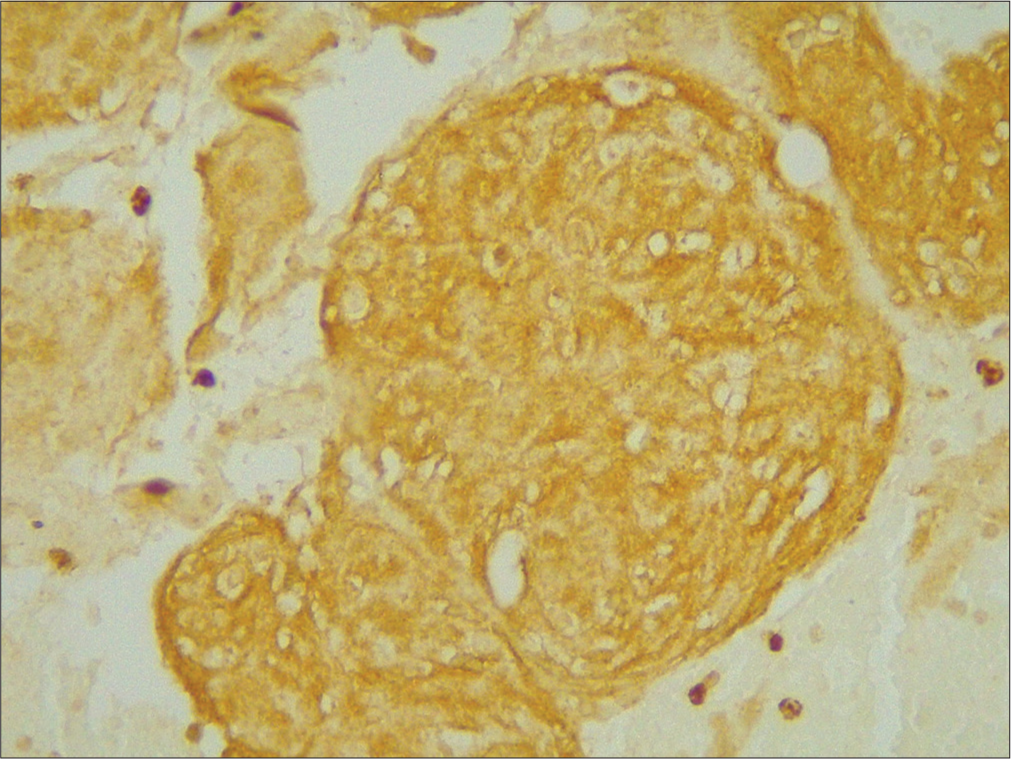
- ×400 p53 strong positive grade 3.
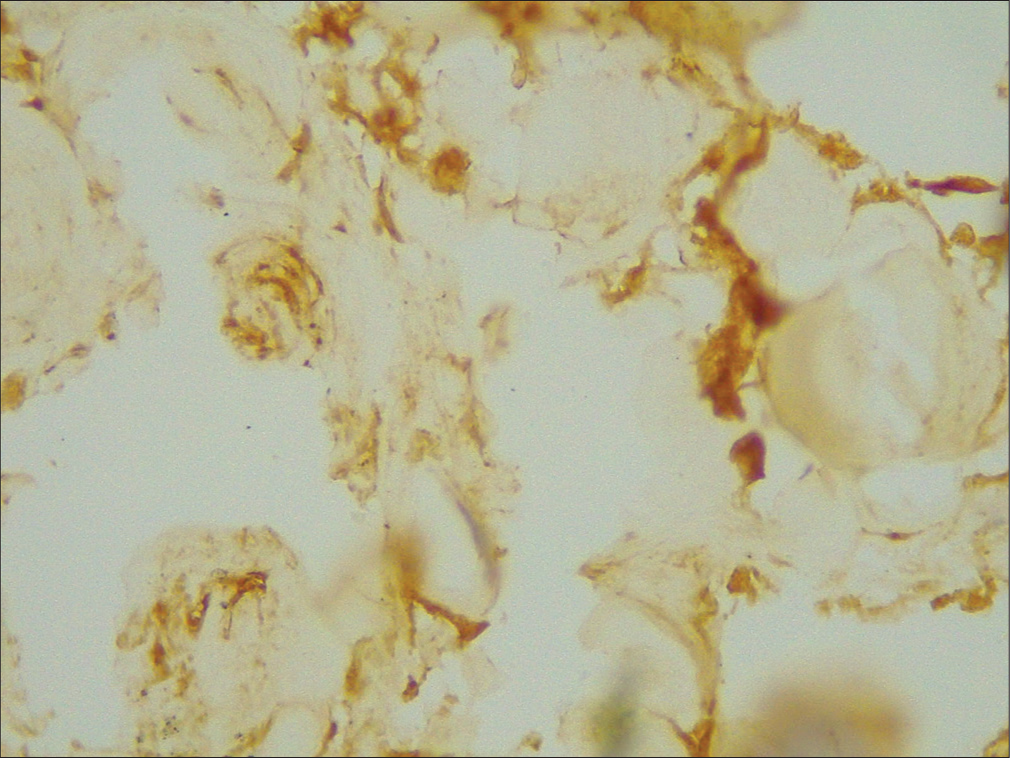
- ×400 p45 weak positive grade 1.
- ×400 p53 intermediate positive grade 2.
The results of the Mann–Whitney test are shown in Table 2. The significance value shows the number was 0.095 or P. Due to P > 0.05, statistically, there was indicative of zero no relationship between merlin expression and orbital meningioma grading. The Mann–Whitney test compared the rankings between groups with negative merlin expression having a higher rank than positive merlin expression (41 vs. 22.07). Since the code for grade 1 is 0, for grade 2 is 1, and for grade 3 is 2, a higher rating indicates a possibly higher grade of orbital meningioma. Thus, what is meant by the relationship here is that grade 3 orbital meningiomas have a tendency to have negative merlin expression compared to positive expression.
| Ekspresi merlin (n, %) | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Nilai (P-value) |
|---|---|---|---|---|
| Negatif | 0 (0) | 0 (0) | 1 (2.3) | 0.095 |
| Positif | 26 (59.1) | 11 (25) | 6 (13.6) | |
| Total | 26 (59.1) | 11 (25) | 7 (15.9) |
Mann–Whitney test P<0.05, the mean expression of Merlin was negative 41%, positive merlin expression 22.07%
The significance value in the Table 3 shows the number was 0.752 or P. Due to P > 0.05, and therefore, statistically, there was no relationship between merlin expression and orbital meningioma grading. The Mann–Whitney test compared the rankings between groups with negative merlin expressions having a higher rank than the positive merlin expression (24.5 vs. 22.45). Since the p53 expression code is negative 0 and the p53 expression is positive 1, a higher rank indicates the possibility of negative merlin expression and a tendency to have positive p53 expression.
| Ekspresi merlin | Ekspresi p53 | Nilai (P-value) | |||||
|---|---|---|---|---|---|---|---|
| Negatif | Positif | Total | |||||
| n | % | n | % | n | % | ||
| Negatif | 0 | 0 | 1 | 2.3 | 1 | 2.3 | 0.752 |
| Positif | 4 | 9.1 | 39 | 88.6 | 43 | 97.7 | |
| Total | 4 | 9.1 | 40 | 90.9 | 44 | 100 | |
Mann–Whitney test P<0.05, the mean expression of merlin was negative 24.5%, positive merlin expression 22.45%
DISCUSSION
Differential diagnoses of meningioma such as excessive arachnoid hyperplasia, fibrous histiocytoma, and solitary fibrous tumor (SFT). Meningothelial hyperplasia can be up to 100 cell layers thick and several millimeters in greatest dimension but has a discontinuous growth pattern and never invades dura, however meningothelial meningioma is usually a single mass. fibrous meningioma versus SFT/hemangiopericytoma: Fibrous meningioma usually show STAT6 negative, and SSTR2a positive but SFT/hemangiopericytoma: usually show STAT6 positive, and SSTR2a negative.[19]
The p53 protein was expressed positively in 78 (49.7%) meningiomas. In 66/141 (46.8%) grade 1 tumors, 11/13 (84.6%) grade 2 tumors, and 1/3 (33.3%) grade 3 tumors, the p53 protein was positively expressed, but there was no significant difference in the relationship between p53 expression and meningioma grade.[20] This study’s findings back up prior research that found no association between p53 expression and the grade of orbital meningioma. This is because the function of p53 may not be related only to the gene that encodes it, but could also depend on alterations in the mechanisms that control p53, such as the tumor suppressor CDKN2A/P16INKa (which encodes p16) and CDKN2B/p15ARF (which encodes p15), where p15 and p16 are as follows: Control of cell cycle development through the checkpoint G1/S phase, and p14ARF (coding p14) as a regulator of cell apoptosis through p53 modulation,[21,22] and it is generally assumed that changes in the p53 gene (mutations) TP53) are rare in meningiomas.[23,24]
Our study showed the positive expression of p53 was about 23.4%. In our study, the positive expression of p53 in all grade. Grade 1 was 59.1%; grade 2, 25%; and grade 3, 15.9%; respectively. High p53 expression in grade 1 is a sign of recurrence in grade 1 meningiomas.[17,25] A study published in Japan in 2021 found an association between p53 expression and meningioma recurrence, in which p53 expression was significantly greater in atypical and anaplastic meningiomas.[26] The presence of 48% methylation of the p53 gene results in p53 gene inactivation, which is significant in the formation and progression of malignant meningioma tumors, including orbital meningioma.[27,28] While previous studies showed that a 1% increase in p53 reported a higher risk of meningioma grade. A study in Croatia found no significant relationship between p53 level and meningioma grade, but it was evident that the mean p53 value increased with tumor grade.[21,29]
Our study’s findings indicated that there was no statistically significant correlation between the grade of meningioma and merlin. This is similar to similarly, a study in Italy in 2007, which reported that there was no relationship between merlin expression with clinicopathological features and histopathological grade, although it tended to decrease from grade 1 to grade 3.[30] These results imply that merlin is not progressive but is involved in the formation of meningioma tumors.[31] There was a similar proclivity for this in our study, and grade 3 orbital meningiomas have a tendency to have a negative merlin expression instead of a positive expression. This is because merlin has a function as the negative regulator of mammal target of rapamycin (mTOR) complex 1, which can be used as a modulator of cell growth and proliferation. If active mTOR will increase protein translation through phosphoinositide three kinase-Akt and cause impaired merlin function, this leads to uncontrolled cell proliferation. This is one of the characteristics that may translate to malignant tumors.[32] Based on this mechanism, the possibility of targeting orbital meningiomas using mTOR inhibitors such as everolimus and vistusertib can be considered.[33] Chronic exposure to epigenetics will stimulate the release of inflammatory mediators, such as cytokines, reactive oxygen species, and reactive nitrogen species so that tumor suppressor genes (merlin and p53) do not work properly. The release of cytokinins, such as interleukin-1β, will cause the activation of the nuclear factor-kappa B pathway, thus increasing mitogen-activated protein kinase in the cytoplasm, which causes an increase in DNMT1 in meningiomas, and DNMT1 and DNMT3b in leptomeningeal cells. NF2 transcription decreases in merlin expression in the cytoplasm which is evident from the brown stain in the can be seen by immunohistochemistry examination. This decrease in merlin expression increases the proliferation of leptomeningeal cells, which will cause orbital meningiomas.[34]
There was no significant link between merlin expression and p53 expression in our study (P = 0.752), which contradicted earlier studies that found a significant relationship between merlin expression and p53 expression in meningiomas. This it is because merlin does not directly activate the p53 promoter at the transcriptional level or induce p53 transcriptional activity through p53-GAL4-DBD fusion under the cytomegalovirus promoter.[20,35] Our study suggests that negative merlin expression in orbital meningiomas tends to have a positive p53 expression. This is consistent with prior research, which found that positive merlin expression in meningiomas had a lower p53.[20] Our results suggest that p53 activity may have an influence on the regulation of merlin expression through the inactivation of NF2.
A p53-dependent increase in transcriptional activity is produced by positive merlin expression, which also enhances p53 stability by preventing Mdm2 protein-mediated p53 degradation as a proto-oncogene. In addition, positive merlin expression may act as a positive regulator of p53 in terms of tumor suppressor gene function and loss of MEG3 expression (as an antiproliferative agent in tumor suppressor that induces p53 activity through transcriptional effects).[12,36-38] The N-terminal merlin is responsible for the stabilization and activation of p53 through the Mdm2 degradation pathway, so there are correlation between p53 and merlin, thus NF2 as a tumor suppressor gene. Overexpression of merlin will induce cell apoptosis and, thus, becomes a positive regulator of p53, thus providing a means in the treatment of orbital meningiomas through overexpression of nonfunctional merlin or Mdm2 with a significance of P = 0.004 where tumor grade significance with significant factor for progression-free survival.[35,39] From our research, it is stated that the presence of merlin and p53 expression can predispose to grading orbital meningioma, and there is an interaction between merlin and p53 as a suppressor of orbital meningioma tumor growth and can be used as a means for the development of orbital meningioma therapy so that it can help as an additional material in the clinical information of orbital meningioma patients.
CONCLUSION
The higher grade (grade 3) has a tendency to express positive p53 and negative merlin, which may potentially play a key role in tumorigenesis of orbital meningioma. In other words, it can be used as an added value for clinical information and behavioral descriptions of orbital meningioma itself. The interaction between merlin and p53 is a tumor suppressor of orbital meningioma and can be used as a means for the potential measure for the development of orbital meningioma therapy. We also highlighted the use of formalin-fixed paraffin block tissue (Formalin-Fixed, Paraffin-Embedded Tissue) to be used for immunohistochemical examination of p53 and merlin as the markers of orbital meningioma predisposition.
Author contribution statement
The project, the primary basic concepts, the proof outline, and the manuscript were all created by Raudatul Janah. The manuscript was reviewed by Daniel Joko Wahyono and Lantip Rujito.
Ethical approval
The General Hospital Research Ethics Commission authorized the procedure for this investigation. Using the registration number LB.02.01/X.6.5/201/2020, Hasan Sadikin Bandung.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Epidemiology of primary intracranial tumours in NW Italy, a population based study: Stable incidence in the last two decades. J Neurol. 2002;249:281-4.
- [CrossRef] [PubMed] [Google Scholar]
- Female predominance in meningiomas can not be explained by differences in progesterone, estrogen, or androgen receptor expression. J Neurooncol. 2006;80:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic profiling by single-nucleotide polymorphism-based array analysis defines three distinct subtypes of orbital meningioma. Brain Pathol. 2015;25:193-201.
- [CrossRef] [PubMed] [Google Scholar]
- Mixed schwannoma with meningioma of the trigeminal nerve. Indian J Pathol Microbiol. 2010;53:769-71.
- [CrossRef] [PubMed] [Google Scholar]
- Relationships between neurofibromatosis-2, progesterone receptor expression, the use of exogenous progesterone, and risk of orbitocranial meningioma in females. Front Oncol. 2019;8:651.
- [CrossRef] [PubMed] [Google Scholar]
- EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383-91.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular and translational advances in meningiomas. Neuro Oncol. 2019;21(Supplement 1):i4-17.
- [CrossRef] [PubMed] [Google Scholar]
- Meningiomas-insights into genetics and correlations with histological features. Arch Clin Cases. 2018;5:20-30.
- [CrossRef] [Google Scholar]
- Genomic analysis of synchronous intracranial meningiomas with different histological grades. J Neurooncol. 2018;138:41-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive molecular profiling identifies FOXM1 as a key transcription factor for meningioma proliferation. Cell Rep. 2018;22:3672-83.
- [CrossRef] [PubMed] [Google Scholar]
- The expression of progesterone receptors in meningiomas of different grades. J Islam Med Dent Coll. 2019;8:65-9.
- [CrossRef] [Google Scholar]
- A role for the p53 pathway in the pathology of meningiomas with NF2 loss. J Neurooncol. 2009;91:265-70.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathological and immunohistochemical evaluation of meningiomas with reference to proliferative markers p53 and Ki-67. J Clin Diagnostic Res. 2016;10:EC15.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of immunohistochemical expression. Leuk Lymphoma. 2014;55:558-64.
- [CrossRef] [PubMed] [Google Scholar]
- Methylation of werner syndrome protein is associated with the occurrence and development of invasive meningioma via the regulation of Myc and p53 expression. Exp Ther Med. 2015;10:498-502.
- [CrossRef] [PubMed] [Google Scholar]
- Abundant immunohistochemical expression of dopamine D2 receptor and p53 protein in meningiomas: Follow-up, relation to gender, age, tumor grade, and recurrence. Braz J Med Biol Res. 2015;48:415-9.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence/regrowth in grade I meningioma: How to predict? Front Oncol. 2020;10:1144.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of 1p and 14q status, MIB-1 labeling index and progesterone receptor immunoexpression in meningiomas: Adjuncts to histopathological grading and predictors of aggressive behavior. Neurol India. 2014;62:376-82.
- [CrossRef] [PubMed] [Google Scholar]
- Meningothelial hyperplasia: A detailed clinicopathologic, immunohistochemical and genetic study of 11 cases. Brain Pathol. 2005;15:109-15.
- [CrossRef] [PubMed] [Google Scholar]
- The significance of immunohistochemical expression of merlin, ki-67, and p53 in meningiomas. Appl Immunohistochem Mol Morphol. 2014;22:46-9.
- [CrossRef] [PubMed] [Google Scholar]
- The significance of immunohistochemical expression of Ki-67, p53, p21, and p16 in meningiomas tissue arrays. Pathol Res Pract. 2008;204:305-14.
- [CrossRef] [PubMed] [Google Scholar]
- Nucleotide variations of TP53 exon 4 found in intracranial meningioma and in silico prediction of their significance. Mol Clin Oncol. 2019;11:563-72.
- [CrossRef] [PubMed] [Google Scholar]
- CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020;140:409-13.
- [CrossRef] [PubMed] [Google Scholar]
- Transitional meningioma benign who grade 1 tumor-A case and review of the literature. IP Arch Cytol Histopathol Res. 2023;8:156-60.
- [CrossRef] [Google Scholar]
- Characterization of morphologically benign biologically aggressive meningiomas. Neurol India. 2009;57:744.
- [CrossRef] [PubMed] [Google Scholar]
- Abstract 4901: p53 expression is a useful predictive marker for recurrence of meningioma. Cancer Res. 2019;79(13 Supplement):4901.
- [CrossRef] [Google Scholar]
- p53 protein expression in meningiomas-A clinicopathologic study of 55 patients. Neurosurg Rev. 2002;25:252-7.
- [CrossRef] [PubMed] [Google Scholar]
- Polymorphisms and DNA methylation of gene TP53 associated with extra-axial brain tumors. Genet Mol Res. 2009;8:8-18.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of Ki-67 and p53 in meningiomas. Neoplasma. 2013;60:480-5.
- [CrossRef] [PubMed] [Google Scholar]
- NF2 gene expression in sporadic meningiomas: Relation to grades or histotypes real time-PCR study. Neuropathology. 2007;27:36-42.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994;6:180-4.
- [CrossRef] [PubMed] [Google Scholar]
- Meningioma genomics: A therapeutic challenge for clinicians. J Integr Neurosci. 2021;20:463-9.
- [CrossRef] [PubMed] [Google Scholar]
- Genomic biomarkers of meningioma: A focused review. Int J Mol Sci. 2021;22:1-13.
- [CrossRef] [PubMed] [Google Scholar]
- The role and regulatory mechanism of IL-1β on the methylation of the NF2 gene in benign meningiomas and leptomeninges. Mol Carcinog. 2016;55:2268-77.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the results of recurrent intracranial meningiomas treated with re-radiosurgery. Clin Neurol Neurosurg. 2017;153:93-101.
- [CrossRef] [PubMed] [Google Scholar]
- Group I Paks as therapeutic targets in NF2-deficient meningioma. Oncotarget. 2015;6:1981.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic/molecular alterations of meningiomas and the signaling pathways targeted. Oncotarget. 2015;6:10671.
- [CrossRef] [PubMed] [Google Scholar]
- Merlin neutralizes the inhibitory effect of Mdm2 on p53. J Biol Chem. 2004;279:7812-8.
- [CrossRef] [PubMed] [Google Scholar]







