Translate this page into:
The potential inflammatory biomarker of bipolar disorder: Neutrophil-to-lymphocyte Ratio – A hospital-based cross-sectional study
*Corresponding author: Rajnish Raj, Department of Psychiatry, Government Medical College, Srinagar, Jammu and Kashmir, India. dr.rajnishrj@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Raj R, Wani ZA, Dar S, Dilawar T, Altaf S, Beigh A, et al. The potential inflammatory biomarker of bipolar disorder: Neutrophil-to-lymphocyte ratio – A hospital-based cross-sectional study. J Neurosci Rural Pract. 2024;15:461-7. doi: 10.25259/JNRP_143_2024
Abstract
Objectives:
Inflammation has been hypothesized as one of the pathophysiological factor for mood disorders. The neutrophil-to-lymphocyte ratio (NLR) has been proposed as a potential peripheral biomarker of mood episodes, as this is an economical and accessible marker of inflammation. This study aims to determine the role of inflammation in the pathophysiology of bipolar disorder (BD) and the potential of NLR as a marker for differentiating mood disorders.
Materials and Methods:
A cross-sectional study was conducted on 195 patients who met the inclusion criteria, of whom 80 were diagnosed with BD (mania), 47 with BD (depression), and 68 with major depressive disorder (MDD). Sociodemographic details and a blood sample were taken for hemogram measures. The NLR and MLR were calculated using the following formula: NLR = neutrophil count/lymphocyte count and MLR = monocyte count/lymphocyte count, and statistical analysis was done.
Results:
The mean age (±standard deviation) of patients with mania, bipolar depression, and MDD were 35.97 (±13.14), 39.27 (±14.28), and 33.41 (±13.21) years, respectively, with an almost equal representation of male and female gender. The NLR ratio of 2.41 ± 0.84 was highest in BD (mania), followed by 1.75 ± 0.41 in bipolar depression and 1.67 ± 0.45 in MDD, which was significant (P < 0.001), while MLR ratio in BD (mania) was highest, followed by MDD and bipolar depression.
Conclusion:
Our result indicates NLR as a marker of differentiation and, thus, strengthens the pathophysiological importance of inflammation in mood disorders.
Keywords
Bipolar disorder
Biomarker
Neutrophil-to-lymphocyte ratio
Mania
Depression
INTRODUCTION
Bipolar disorder (BD) is a psychiatric illness characterized by periods of mania, hypomania, or depression. It is acute in onset and may be characterized by a single episode, but is usually recurrent in frequency, leading to significant impairment in social, personal, and occupational functions of daily life. As per the National Mental Health Survey (2015–2016), conducted across 12 states of our country and supported by the Government of India, the lifetime prevalence of BD was 0.5% in India[1] and 2.4% worldwide.[2] According to data from the World Health Organization’s World Mental Health Surveys, Major depressive disorder (MDD) and BD, have the greatest effect on the duration of functional impairment in individuals.[3]
There are numerous factors that have been implicated in the pathophysiology of BD. These include but are not restricted to hypothalamic-pituitary-adrenal axis dysregulation, neurotransmitter imbalance, changes in monoamine and glutaminergic transmission, inflammatory pathways, and immune system dysregulation.[4-7]
The immune hypothesis first proposed that the improvement in BD is due to immunomodulatory effect of lithium.[8] Since then, various studies have been done to find the role of inflammation and immune imbalance in the development of BD.[9-11]
A study review has indicated an increased level of proinflammatory cytokines and chemokines along with a reduction in anti-inflammatory cytokines during manic and depressive episodes, leading to the possible role of inflammation in BD.[12]
Further, diving into the inflammatory hypothesis, neutrophils, lymphocytes, monocytes, and platelets play a key role in it. Neutrophils are white blood cells (WBCs) involved in innate immunity, whereas lymphocytes in adaptive immunity and monocytes exhibit proinflammatory effects against inflammatory diseases.[13,14] Platelets contain serotonin, which gets activated by inflammation and stimulates the release of neutrophils, lymphocytes, monocytes, and cytokines; thus, it has been used as a peripheral model of receptor-mediated signal transduction,[15,16] which makes neutrophilto-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) of special concern, as it represents a rapid and economical form of investigation. Several studies have been done to evaluate NLR, MLR, and PLR as potential biomarkers for BD, with findings of increased NLR in BD when compared with healthy controls.[17] Some studies have found increased PLR in suicide attempts.[18] A meta-analysis has shown increased NLR and PLR in BD compared to control. It was the first time that Mazza et al.[19] evaluated blood cell inflammatory indicators in BD and reported increased levels of both NLR and MLR in mania compared to depressive episodes. All these findings indicate the certainty of the inflammatory hypothesis.
Considering the above facts and findings, this study is being done to evaluate the role of NLR across Bipolar disorder -Mania (BD-Mania), Bipolar disorder -Depression (BD-Depression), and Major Depressive Disorder (MDD).
MATERIALS AND METHODS
This research was cross-sectional in design and was conducted at the Institute of Mental Health and Neurosciences-Kashmir (An Associated Hospital of Government Medical College, Srinagar) among visiting outpatients and inpatients diagnosed with BD (mania or depression) and Major Depressive Disorder (MDD). This study was conducted from November 2023 to March 2024. The convenience sampling technique was used for recruiting the cases after fulfilling the inclusion criteria. The study was performed after obtaining written informed consent from all participants or their attendants. The Institutional Review Board of Government Medical College and Associated Hospitals, Srinagar, Jammu and Kashmir, India, approved this research study. The strengthening the reporting of observational studies in epidemiology (STROBE) guidelines were followed in the conduct of the study.
For this study, patients aged 18 years or older, diagnosed with BD (current episode: Mania or depression), and MDD diagnosed as per the Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5), and those who gave written informed consent were taken. Patients who were pregnant or had recent childbirth in the past three months, had severe and uncontrolled medical comorbidities such as type 2 diabetes mellitus, hypertension, and untreated hypothyroidism were excluded from the study. The set criteria for uncontrolled type 2 diabetes mellitus were a level of more than 126 mg/dL of fasting blood glucose,[20] while uncontrolled hypertension was defined as a blood pressure of more than 140 mmHg systolic and more than 90 mmHg diastolic pressure despite taking antihypertensive medications, in diagnosed patients of hypertension.[21] Patients who were on corticosteroids, antibiotics or clozapine prescriptions in the past six months, had a current substance use disorder excluding tobacco or refused to give informed written consent were also excluded from this study.
Individual interview sessions were held to collect socio-demographic details: age, gender, family type, current episode, duration of illness, family history of mood disorders, and current treatment status. For blood parameters, a sample was taken from the median cubital vein from either arm and collected in ethylenediaminetetraacetic acid vacutainers. Samples were processed in our hospital laboratory under the Sysmex Fully Automatic XN1000. The principle of fluorescence flow cytometry is behind the estimation of different types of WBC. Neutrophil, lymphocyte, monocyte, and platelet counts were collected, and ratios were calculated using the following formulas:
NLR = neutrophil count/lymphocyte count, and MLR = monocyte count/lymphocyte count.
We summarized categorical variables using percentages and continuous variables as mean and standard deviation (SD). The duration of illness was summarized as the median and interquartile range (IQR). A one-way analysis of variance (ANOVA) was used to test the difference in NLR across the three groups. A multivariate linear regression analysis model was used to estimate the difference in NLR across the three groups, adjusted for age, gender, psychotic features, and treatment status. Analysis was done using Stata version 17. Two-sided p-values are reported, and P < 0.05 were considered statistically significant.
RESULTS
Two hundred and thirty patients with a diagnosis of BD or MDD were approached to participate in this study, of whom ten had uncontrolled medical comorbidities such as diabetes mellitus and hypothyroidism; five patients were on antibiotics; eight patients had comorbid substance use; and 12 patients were not willing to participate in this study. A total of 195 patients provided written informed consent and were taken for the study. A total of 80 patients were in Mania; 47 of them had bipolar depression, and 68 had MDD. The mean age (±SD) of patients with mania, bipolar depression, and MDD were 35.97 (±13.14), 39.27 (±14.28), and 33.41 (±13.21) years, respectively.
A total of 94 males and 101 females participated in the study, out of which 23.5% (n = 46) had psychotic features. There was a positive family history of mood disorder in 35.8% (n = 70) of the patients. More than half of the patients, 59.4% (n = 116), were already on pharmacological treatment. Complete sociodemographic details of patients are mentioned in Table 1. As the distribution of duration of illness in our study population was skewed, we presented it as median (IQR).
| BD (Mania) | BD (Depression) | MDD | |||
|---|---|---|---|---|---|
| Age, Mean (±SD) | 35.97 (±13.14) | 39.27 (±14.28) | 33.41 (±13.21) | ||
| Duration of illness (years), Median (IQR) | 6 (2–10) | 5.2 (2.6–10) | 2.9 (0.95–5) | ||
| n (%) | n (%) | n (%) | Chi-square | P-value | |
| Age group | |||||
| <30 years | 35 (43.75) | 18 (38.29) | 37 (54.41) | 3.40 | 0.49 |
| 31–50 years | 33 (41.25) | 20 (42.55) | 22 (32.35) | ||
| >50 years | 12 (15) | 9 (19.14) | 9 (13.23) | ||
| Gender | |||||
| Male | 49 (61.25) | 16 (34.04) | 29 (42.64) | 10.06 | 0.007 |
| Female | 31 (38.75) | 31 (65.95) | 39 (57.35) | ||
| Family type | |||||
| Nuclear | 39 (48.75) | 21 (44.68) | 38 (55.88) | 3.95 | 0.41 |
| Extended nuclear | 3 (3.75) | 5 (10.63) | 5 (7.35) | ||
| Joint family | 38 (47.50) | 21 (44.68) | 25 (36.76) | ||
| Family history of mood disorder, present | 32 (40) | 13 (27.65) | 25 (36.76) | 1.99 | 0.36 |
| Psychotic features | 26 (32.50) | 9 (19.14) | 11 (16.17) | 6.11 | 0.04 |
| Was on treatment | 43 (53.75) | 39 (82.97) | 34 (50) | 14.39 | 0.001 |
SD: Standard deviation, IQR: Interquartile range, BD: Bipolar disorder, MDD: Major depressive disorder
Regarding laboratory parameters, WBC values were highest in mania, followed by bipolar depression and MDD. A similar trend was observed in neutrophil count. Further, the highest NLR of 2.41 ± 0.84 was observed in mania, followed by bipolar depression, having 1.75 ± 0.41, and values of 1.67 ± 0.45 in MDD. The MLR values were also highest in mania, followed by MDD and BD-depression (0.33 ± 0.14 > 0.27 ± 0.10 > 0.26 ± 0.10, respectively). Both ratios were statistically significant, that is, NLR (P < 0.001) and MLR (P = 0.006). A graphical representation of the NLR mean across mood disorders is displayed in Figure 1. Complete laboratory measurements were analyzed using ANOVA, as shown in Table 2.
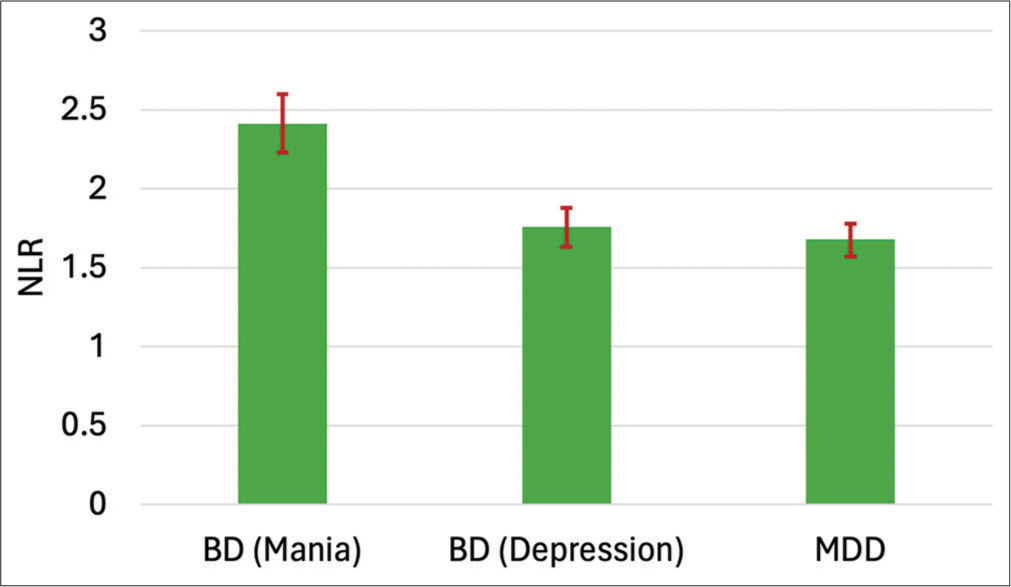
- Bar graph depicting the mean of neutrophil-to-lymphocyte ratio (NLR) across mood disorders. The error bars indicate a 95% confidence interval. BD: Bipolar disorder, MDD: Major depressive disorder.
| Parameter | Total sample | BD (Mania) | BD (Depression) | MDD | Chi-square | F-value | P-value |
|---|---|---|---|---|---|---|---|
| WBC (103 cells/mm3) | 5.82 (1.59) | 6.20 (1.76) | 5.63 (1.55) | 5.52 (1.30) | 6.46 | 3.90 | 0.039 |
| Neutrophil, (103cells/mm3) | 3.54 (1.33) | 3.96 (1.52) | 3.29 (1.16) | 3.21 (1.04) | 11.11 | 7.39 | 0.004 |
| Lymphocyte (103 cells/mm3) | 1.85 (0.59) | 1.70 (0.53) | 1.92 (0.63) | 1.97 (0.60) | 1.66 | 4.48 | 0.435 |
| Monocyte (103 cells/mm3) | 0.51 (0.19) | 0.53 (0.20) | 0.49 (0.19) | 0.51 (0.17) | 2.25 | 0.83 | 0.324 |
| Platelet (103 cells/mm3) | 173.2 (70.58) | 174.68 (66.39) | 162.74 (64.46) | 178.67 (79.14) | 3.15 | 0.74 | 0.206 |
| Neutrophil-to-Lymphocyte Ratio | 1.99 (0.72) | 2.41 (0.84) | 1.75 (0.41) | 1.67 (0.45) | 40.05 | 29.15 | <0.001 |
| Monocyte-to-lymphocyte ratio | 0.29 (0.12) | 0.33 (0.14) | 0.26 (0.10) | 0.27 (0.10) | 10.12 | 5.07 | 0.006 |
WBC: White blood cell, ANOVA: Analysis of variance, BD: Bipolar disorder, MDD: Major depressive disorder
The difference in NLR across BD (Mania), BD (Depression), and MDD using multivariate regression analysis model is estimated in Table 3, and it depicts NLR in BD (Mania) was significantly greater than the other two groups after adjusting for age, gender, psychotic features, and treatment status (P < 0.001).
| Diagnosis | Margin | Standard error | t | 95% confidence interval | P-value | |
|---|---|---|---|---|---|---|
| BD (Mania) | 2.39 | 0.07 | 33.00 | 2.24 | 2.53 | Reference group |
| BD (Depression) | 1.75 | 0.09 | 18.27 | 1.56 | 1.94 | <0.001 |
| MDD | 1.69 | 0.07 | 21.67 | 1.54 | 1.85 | <0.001 |
NLR: Neutrophil-to-lymphocyte ratio, BD: Bipolar disorder, MDD: Major depressive disorder, t: Test statistics
DISCUSSION
The results of our study showed that there is increased inflammatory activity, as evidenced by increased NLR and MLR in Mania, compared to bipolar depression and MDD. Apart from this, patients with BD had higher NLR than MDD, thus indicating NLR as an independent biomarker for bipolar depression and MDD. The previous studies done in Italy and Romania have come up with similar findings.[19,22] To the best of our knowledge, this study was the first attempt from India to replicate such findings.
At present, there is growing interest regarding the inflammatory hypothesis in mood disorder onset and progression. Studies have shown that increased cytokine levels, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-2, IL-6, and interferon gamma (IFN-γ), increase the catabolism of tryptophan.[23] It activates the guanosine-triphosphate-cyclohydrolase 1 enzyme, leading to the formation of neopterin,[24-26] instead of tetrahydrobiopterin, which is required as a co-factor by phenylamine hydroxylase, tyrosine hydroxylase, and tryptophan hydroxylase for the formation of various neurotransmitters such as dopamine, norepinephrine, and serotonin.[27-29] Thus, inflammatory cytokines lead to decreased synthesis of these monoamines. Cytokines also lead to microglial activation, leading to the formation of pro-oxidant and reactive oxygen species (i.e., 8-hydroxy-2-deoxyguanosine, F2-isoprostanes, and malondialdehyde) and reduced anti-oxidants (such as glutathione, superoxide dismutase, and glutathione peroxidase),[30] further disrupting the neurocircuitry connecting the pre-frontal cortex, amygdala, hippocampus, insula, and anterior cingulate cortex, which actually regulates mood and cognitive functions.[31-33] These inflammatory cytokines can also cause impairment in the hypothalamic-pituitary axis due to decreased glucocorticoid receptor synthesis and reduced sensitivity in the pituitary and hypothalamus.[34] Cytokines cause an increase in glutamate levels, leading to calcium influx through N-methyl-D-aspartate receptors, causing neurotoxicity and decreased neuroplasticity.[35] Recent interest has been growing in the gut-brain axis, which involves the parasympathetic nervous system, gut endocrine system, circulatory system, and cytokines.[36] It has been hypothesized by Hamdani et al.[37] that the production of proinflammatory cytokines from the gastrointestinal system can lead to manic episodes. Furthermore, disorders such as inflammatory bowel disease, systemic lupus erythematosus, autoimmune thyroiditis, Myocardial infarction, stroke, atherosclerosis, hypertension, diabetes mellitus, dyslipidemia, gout, Gullain–Barre syndrome, autoimmune hepatitis, rheumatoid arthritis, multiple sclerosis, psoriasis, chronic infections like toxoplasmosis, and other viral infections are associated with BD.[7]
Various studies have been done based on this concept to understand the role of different anti-inflammatory drugs in managing mania and depression, namely, celecoxib (NSAIDs), omega-3 fatty acids, statin, and N-acetyl cysteine, with the latter having the strongest evidence due to its use in the treatment of bipolar depression.[38-41] It has also been seen that IFN-based therapy can induce neuroinflammation, resulting in manic and depressive symptoms, whereas in a randomized control trial, infliximab, a TNF-α inhibitor, has been used with response in treatment-resistant depression.[42,43] Conventional mood stabilizers have shown a mixed anti-inflammatory and proinflammatory effect; thus, their effects remain unclear,[44,45] whereas commonly used atypical antipsychotics in BD have been shown to alter the NLR ratio, specifically reducing it,[46] thus making a significant impact on the improvement of symptoms.
In line with the previous study by Mazza et al.,[19] our study found the highest NLR in BD-Mania, followed by BD-depression, and then MDD, which is statistically significant (P < 0.001). Regarding another significant finding of MLR (P = 0.006) in our study, which is highest in BD (Mania), between BD (Depression) and MDD, it is somewhat on the upper side in MDD (0.27) compared to BD-depression (0.26), which was contrary to the previous study reported by Koureta et al.[47] with the finding of increased MLR in BD (Depression) than unipolar depression. This difference can be explained by a different sub-group of the population which is distinct from the one used by Koureta et al.,[47] as MLR has been found to vary with age and gender.[48,49]
Our study has several limitations. First, we did not assess other important biological markers related to the immune system, such as IL-1β, TNF-α, IL-2, IL-6, IL-10, IL-12, stnfr, KLOTHO, and C-reactive protein values, as these markers are affected by different mood states such as mania or depression.[50,51] Second, we did not evaluate the severity of manic or depressive symptoms in comparison to the NLR ratio; the previous studies have shown an association between the NLR ratio and severity.[52,53] Third, we did not include a healthy euthymic control sample in this study; therefore, no causal modeling is possible. Fourth, more than half of the patients were already on pharmacological treatment, including lithium, which can alter inflammatory biomarkers and bias results. Fifth, physiological markers such as body mass index, eating habits, and lifestyle routines, were not taken into consideration. A longitudinal follow-up study in the future could be done to further strengthen this analysis, keeping in mind these limitations.
CONCLUSION
This study highlights the role of inflammation in the pathophysiology of mood disorders and demonstrates its significance on a practical basis, as routine complete blood counts are an economical, rapid, and convenient means to serve as a potential biomarker for mood episodes and their various subtypes. Future research is needed to identify cheap and economical biomarkers, which are valid and specific.
Acknowledgments
The authors would like to thank all the nursing staff and laboratory technicians for their continued assistance in blood sample collection, analysis, and prompt reporting.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors have contributed significantly to the study design, data collection and manuscript preparation.
Ethical approval
This study was approved by the Institutional Review Board of Government Medical College and Associated hospitals, Srinagar, Jammu and Kashmir, India, number : IRBGMC/PSY 358, dated 29/11/2023.
Declaration of patient consent
A written informed consent was obtained from all participants after explaining them the objectives of the study and ensuring complete anonymity.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The National Mental Health Survey of India (2016) Prevalence, socio-demographic correlates and treatment gap of mental morbidity. Int J Soc Psychiatry. 2020;66:361-72.
- [CrossRef] [PubMed] [Google Scholar]
- Improving functioning, quality of life, and well-being in patients with bipolar disorder. Int J Neuropsychopharmacol. 2019;22:467-77.
- [CrossRef] [PubMed] [Google Scholar]
- Days out of role due to common physical and mental conditions: Results from the WHO World Mental Health surveys. Mol Psychiatry. 2010;16:1234-46.
- [CrossRef] [PubMed] [Google Scholar]
- The neurobiology of depression: An integrated view. Asian J Psychiatry. 2017;27:101-11.
- [CrossRef] [PubMed] [Google Scholar]
- Pathways underlying neuro progression in bipolar disorder: Focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804-17.
- [CrossRef] [PubMed] [Google Scholar]
- Bipolar disorder and immune dysfunction: Epidemiological findings, proposed pathophysiology and clinical implications. Brain Sci. 2017;7:144.
- [CrossRef] [PubMed] [Google Scholar]
- A biochemical basis for the actions of lithium on behaviour and on immunity: Relapsing and remitting disorders of inflammation and immunity such as multiple sclerosis or recurrent herpes as manic-depression of the immune system. Med Hypotheses. 1981;7:891-905.
- [CrossRef] [PubMed] [Google Scholar]
- An immune gate of depression-early neuroimmune development in the formation of the underlying depressive disorder. Pharmacol Rep. 2019;71:1299-307.
- [CrossRef] [PubMed] [Google Scholar]
- Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroinflammation and psychiatric illness. J Neuroinflamm. 2013;10:43.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J Psychiatr Res. 2017;92:160-82.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-tolymphocyte ratio predicting suicide risk in euthymic patients with bipolar disorder: Moderatory effect of family history. Compr Psychiatry. 2016;66:87-95.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil: A cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113.
- [CrossRef] [PubMed] [Google Scholar]
- Role of platelets in neuroinflammation: A wide-angle perspective. J. Neuroinflammation. 2010;7:10.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet haemostatic function in psychiatric disorders: Effects of antidepressants and antipsychotic drugs. World J Biol Psychiatry. 2016;18:564-74.
- [CrossRef] [PubMed] [Google Scholar]
- Increased neutrophil/lymphoctye ratio in patients with bipolar disorder: A preliminary study. Psychiatr Danub. 2015;27:180-4.
- [Google Scholar]
- High-lethality of suicide attempts associated with platelet to lymphocyte ratio and mean platelet volume in psychiatric inpatient setting. World J Biol Psychiatry. 2021;22:119-27.
- [CrossRef] [PubMed] [Google Scholar]
- Cross-sectional study of neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratios in mood disorders. Gen Psychiatry. 2019;58:7-12.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62-7.
- [CrossRef] [PubMed] [Google Scholar]
- Uncontrolled hypertension, treatment, and predictors among hypertensive out-patients attending primary health facilities in Johannesburg, South Africa. Healthcare (Basel). 2023;11:2783.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-to-lymphocyte ratio, a novel inflammatory marker, as a predictor of bipolar type in depressed patients: A quest for biological markers. J Clin Med. 2021;10:1924.
- [CrossRef] [PubMed] [Google Scholar]
- Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906-14.
- [CrossRef] [PubMed] [Google Scholar]
- Increased autoimmune activity against 5-HT: A key component of depression that is associated with inflammation and activation of cell-mediated immunity, and with severity and staging of depression. J Affect Disord. 2012;136:386-92.
- [CrossRef] [PubMed] [Google Scholar]
- Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175-87.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma soluble interleukin-2-receptor in depression: Relationships to plasma neopterin and serum IL-2 concentrations and HPA-axis activity. Eur Psychiatry. 1995;10:397-403.
- [CrossRef] [PubMed] [Google Scholar]
- Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neurosci Biobehav Rev. 2016;64:148-66.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of noradrenergic function by inflammatory cytokines and depolarization. J Neurochem. 2003;86:774-83.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: Role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175-82.
- [CrossRef] [PubMed] [Google Scholar]
- The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:134-44.
- [CrossRef] [PubMed] [Google Scholar]
- Microglial dysregulation in psychiatric disease. Clin Dev Immunol. 2013;2013:608654.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroinflammation in bipolar disorder-A [(11)C]-(R)-PK11195 positron emission tomography study. Brain Behav Immun. 2014;40:219-25.
- [CrossRef] [PubMed] [Google Scholar]
- Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr Opin Psychiatry. 2013;26:19-26.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86-105.
- [CrossRef] [PubMed] [Google Scholar]
- Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J Neural Transm. 2014;121:925-32.
- [CrossRef] [PubMed] [Google Scholar]
- Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701-12.
- [CrossRef] [PubMed] [Google Scholar]
- Resolution of a manic episode treated with activated charcoal: Evidence for a brain-gut axis in bipolar disorder. Aust N Z J Psychiatry. 2015;49:1221-3.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: A systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91:21-32.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: Meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139:404-19.
- [CrossRef] [PubMed] [Google Scholar]
- N-acetyl cysteine for depressive symptoms in bipolar disorder-A double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468-75.
- [CrossRef] [Google Scholar]
- Maintenance N-acetyl cysteine treatment for bipolar disorder: A double-blind randomized placebo controlled trial. BMC Med. 2012;10:91.
- [CrossRef] [PubMed] [Google Scholar]
- Interferon alpha (ifnα) and psychiatric syndromes. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731-46.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31-41.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of lithium on inflammation. ACS Chem Neurosci. 2014;5:451-8.
- [CrossRef] [PubMed] [Google Scholar]
- Review of lithium effects on immune cells. Immunopharmacol Immunotoxicol. 2015;37:111-25.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of antipsychotic treatment on neutrophil-to-lymphocyte ratio during hospitalization for acute psychosis in the course of schizophrenia-a cross-sectional retrospective study. J Clin Med. 2022;11:232.
- [CrossRef] [PubMed] [Google Scholar]
- Immune cell ratios are higher in bipolar affective than unipolar depressive disorder and modulated by mood episode: A retrospective, cross-sectional study. Brain Sci. 2023;13:448.
- [CrossRef] [PubMed] [Google Scholar]
- Normal reference intervals of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and systemic immune inflammation index in healthy adults: A large multi-center study from Western China. Clin Lab. 2019;65:255-65.
- [CrossRef] [PubMed] [Google Scholar]
- High neutrophillymphocyte ratio in schizophrenia independent of infectious and metabolic parameters. Nord J Psychiatry. 2018;72:336-40.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine profiles in bipolar affective disorder: Focus on acutely ill patients. J Affect Disord. 2006;90:263-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009;116:214-7.
- [CrossRef] [PubMed] [Google Scholar]
- Increased neutrophil/lymphocyte ratio in patients with depression is correlated with the severity of depression and cardiovascular risk factors. Psychiatry Investig. 2016;13:121-6.
- [CrossRef] [PubMed] [Google Scholar]
- Increased neutrophil-lymphocyte ratios in depressive adolescents is correlated with the severity of depression. Psychiatry Res. 2018;268:426-31.
- [CrossRef] [PubMed] [Google Scholar]







