Translate this page into:
Comparing distributed versus massed practice on functional recovery and Brain-Derived Neurotrophic Factor (BDNF) in acute stroke subjects
*Corresponding author: Dr. K. Vijaya Kumar, Department of Physiotherapy, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education (MAHE), Manipal, India. vijay.kk@manipal.edu
-
Received: ,
Accepted: ,
How to cite this article: Kate M, Kumar V, Nayak A, Shirali A. Comparing distributed versus massed practice on functional recovery and Brain-Derived Neurotrophic Factor (BDNF) in acute stroke subjects. J Neurosci Rural Pract. 2024;15:238-44. doi: 10.25259/JNRP_416_2023
Abstract
Objectives:
Globally, stroke is known to be one of the major health problems, resulting in disability among an aging population. Rehabilitation is a process of re-learning of skills, lost due to brain injury. Many factors influence motor learning post neurological insult and practice is one of the key factors which influence relearning or reacquisition of lost motor skills. Practice can be varied concerning order (blocked or random), scheduling (massed or distributed), or whole and part practice. The study observed the effect of variations in practice schedules on motor and functional recovery.
Materials and Methods:
Thirty-two acute stroke subjects were recruited and equally divided into two groups (16 in massed and 16 in distributed). Both groups received an accelerated skill acquisition program (ASAP) for six sessions a week for 2 weeks. Pre- and post-outcome measures included stroke rehabilitation assessment of movement (STREAM) for motor recovery, modified Barthel index (MBI) for functional recovery, and brain-derived neurotrophic factor (BDNF) for neuroplasticity.
Results:
The median scores of participants in the massed practice group before the intervention, of STREAM total, MBI, and BDNF were 23.5, 19, and 0.65, respectively, whereas post values of STREAM total, MBI, and BDNF were 40.5, 60.5, and 0.75, respectively. The median scores of the distributed practice group of the pre-STREAM total, MBI, and BDNF were 23.5, 6.5, and 0.70, respectively, whereas the post-STREAM total, MBI, and BDNF were 41, 45.5, and 0.80, respectively. P-value was reported to be <0.05 while comparing pre- and post-values of STREAM, MBI, and BDNF within both intervention groups. The median change scores of STREAM, MBI, and BDNF reported P ≥ 0.05 when compared between the groups.
Conclusion:
Both the groups had significant recovery post-intervention designed based on ASAP, about impairment mitigation, pursuing skilled movement leading to significant functional gains. Appropriate timing along with optimal dosage became an active ingredient in functional recovery in acute stroke subjects. The distributed practice might have added effect of spacing, resulting in easier learning and accuracy of skills. The study reveals that distributed practice can be part of regular clinical practice to enhance functional recovery in acute stroke rehabilitation.
Keywords
Acute stroke
Practice
Motor skills
Neuroplasticity
Physical therapy
INTRODUCTION
Stroke is the second most common cause of mortality in the world and is a serious health issue on a global scale.[1] The mainstay of the treatment for stroke survivors to recover from lingering motor deficits is a rehabilitation program.[2]
Improved arm-hand functions, gait and mobility, and activities of daily living (ADL) activities are the results of novel rehabilitation strategies involving diverse physiotherapy treatment modalities such as early mobilization, balance training, task-oriented training, and gait training.[3,4] The accelerated skill acquisition program (ASAP), an evidence-based intervention, was created with modern motor learning concepts to address post-stroke deficits.[5,6] Brain-derived neurotrophic factor (BDNF), which is known to prevent neuronal cell deaths during cerebral ischemia and to further improve synaptic and axonal plasticity associated with learning, memory, and sensori-motor recovery, is a crucial component in controlling neuroplastic changes in the brain.[7] The physical activity raised BDNF concentrations in studies using animal stroke models, according to Alcantara et al.’s review, which also called for greater research on human BDNF responses to exercise training.[8]
Neuro-rehabilitation following a stroke is a process of re-learning and skill acquisition.[9] Practice is a crucial part of motor learning, and its variables – such as quantity, variety, and schedule – ensure that movement quality, function, and timing improve in line with increased movement capabilities.[9] In distributed practice, the exercises are interspersed with longer rest intervals than the entire duration of exercises, whereas in massed practice, all exercises must be performed, which limits the rest within the sessions.[10] Massed and distributed scheduling of practice demonstrates benefits in several types of skills, including continuous and discrete tasks, respectively.[11]
The diversity of practice in acute stroke rehabilitation was less well-studied because it is the most important part of motor learning. Studies on the impact of mobility training and practice schedules on motor and functional recovery during early stroke rehabilitation are lacking. To examine the effects of massed versus distributed practice on functional recovery and BDNF in acute stroke rehabilitation, this study was conducted.
MATERIALS AND METHODS
Study design and participants
The study was carried out in Mangalore’s KMC Hospital’s physiotherapy division, a tertiary care facility. Comparing the two practice schedules, such as mass practice and distributed practice, in a non-randomized therapeutic group experiment. The study was carried out between December 2018 and March 2020.
We sought out individuals with acute (<2 weeks) ischemic or hemorrhagic stroke in the supra-tentorial region who were older than 18 years old, had just one episode of stroke, and had a recovery period of 2–10 days. Inclusion criteria for the study comprised people with a Brunnstrom recovery stage (BRS) of ≥2, a modified Rankin score (MRS) ranging from 2 to 5, and a Mini-Mental State Examination (MMSE) score of ≥23.[12] Exclusion criteria included subjects with an infratentorial cerebrovascular accident, stroke patients with concurrent neurological diseases such as Alzheimer’s or Parkinson’s, as well as the presence of any unilateral neglect, Pusher syndrome, a severe visual impairment such as hemianopia or other perceptual deficits, and musculoskeletal conditions that could affect performance. The study protocol was approved by the Scientific Committee and Institutional Ethics Committee at KMC Mangalore, Manipal Academy for Higher Education. The study was registered under the Clinical Trial Registry-India with reference no. REF/2018/12/023129. All the participants who participated in the study were provided with information regarding the rehabilitation program, and informed consent was undertaken from them before participation.
Non-randomization and blinding
The research was set up in a parallel group. The study was set up as a non-randomized experiment in a parallel group. A.N., a member of the research team who was blind to the assessment and intervention, randomly assigned 16 volunteers to each of the two intervention groups (massed practice group and distributed practice group) for our study, which enrolled 32 acute stroke patients. A.N., who was in charge of allocating funds, was not engaged in the selection of participants, their evaluation, or the delivery of intervention programs. The assessment and intervention plans for the participants were organized by V.K. and M.K. A description of the process is provided in Figure 1.
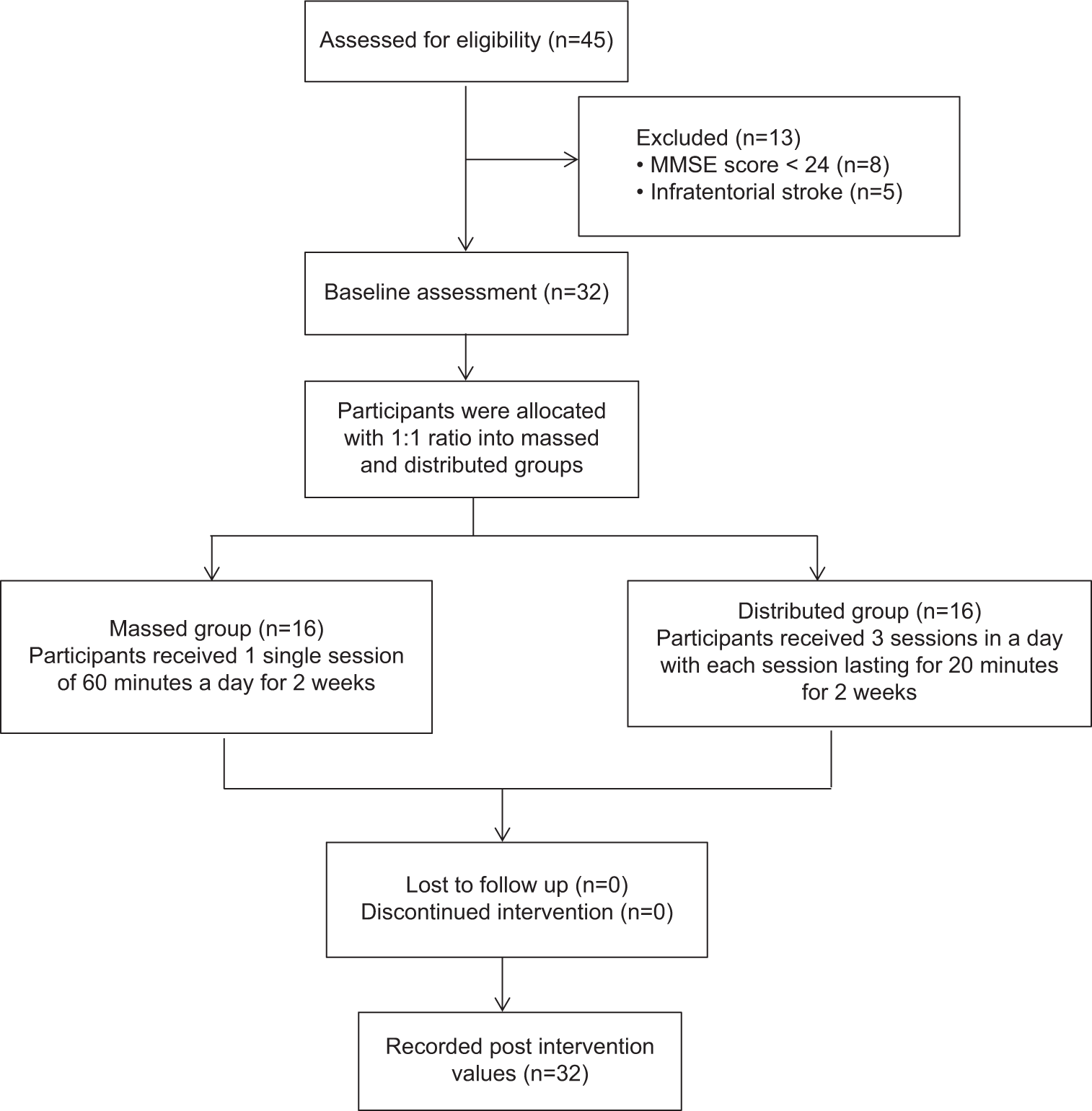
- Participant flowchart. MMSE: Mini-mental state examination.
Intervention
For the study, the treatment strategy was modified from the ASAP, an evidence-based intervention targeting the re-learning of skills to best influence brain plasticity. Identifying the performance threshold, addressing modifiable impairments, ensuring a challenging and meaningful environment, maintaining goal-directedness in the movement organization, artificially breaking down tasks, ensuring active participation, opportunities for self-direction, and balancing between immediate and long-term needs are the eight fundamental principles on which it is based.[13] One of the major principles of ASAP is the practice component, which uses the overload and specificity principles to increase motor capacity.
For paretic extremities
Through the routine examination process, impairments such as weakness and altered tone were discovered. The affected muscles of the bigger joints, such as the hip, knee, and ankle in the lower extremity (LE), as well as the shoulder, elbow, and wrist in the upper extremity (UE), were actively practiced at the bedside to address the weakness. On the basis of the overload and specificity theory, it was further advanced by adding weights while training. The movements learned were subsequently transferred into functional tasks with clear goals, including holding a bottle or doing a reach-out exercise for LE, which required the participant to change both the position of his feet on the ground and in the air. Weight-bearing activities performed repeatedly while seated and standing led to the normalization of tone.
Mobility training
To ascertain the patients’ threshold for trunk mobility, bridging exercises like transitioning frequently from supine to side-lying to sitting posture were given to the patients. They were, then, forced to stand after completing these motions, initially with help and eventually with less help. The individual was encouraged to participate in mobility exercises and helped with problem-solving involving identifying bad posture and uneven weight-bearing when standing. The participant learned self-directed ways to alter their maladaptive postures through visual and audible feedback, which aided in the healing process.
Massed group (MG)
Massed practice was performed on the subjects assigned to this group. Every day, they had one therapy session that lasted between 50 and 60 min. Exercises for the UE, trunk, and LE were given to the subjects in a blocked way during each session.
Distributed group (DG)
This group’s subjects underwent scattered practice. They had three therapy sessions in total each day, with 3 h in between. Exercises for the UE, trunk, and LE were given to the subjects in a random order, with each session lasting about 15–20 min.
Outcome measures
Before and after the intervention, baseline measurements were taken using the Stroke Rehabilitation Assessment of Movement (STREAM) including UE, LE, basic mobility components, and its total score; Modified Barthel Index (MBI) and BDNF values by senior physiotherapist VK, an independent blinded assessor with 15 years of experience in stroke rehabilitation.
STREAM is a measurement technique that counts voluntary movement and fundamental mobility after a stroke. It consists of three domains: Basic mobility, UE, and LE voluntary movement. Basic mobility is evaluated out of 30, while the UE and LE components are each given a score out of 20. It has been reported to have a weighted value ranging from 0.55 to 0.94 with good to outstanding inter-rater reliability. The STREAM UE, LE, and basic mobility subscales exhibit strong contemporaneous validity with the Fugl-Meyer Upper Extremity, Fugl-Meyer Lower Extremity, and Rivermead Mobility Index, respectively. The Barthel Index (BI) and FM have strong positive correlations with the convergent validity of the total STREAM score.[24]
MBI is a gauge of ADL independence. It has a 5-point system and 10-part structure. Excellent concurrent validity with BI among stroke patients is demonstrated by the Performance Evaluation tool, MBI, as well as excellent inter- and intra-rater reliability.[15]
1.5 mL of blood was drawn under aseptic settings from the subject’s antecubital veins by the hospital’s nursing staff both before and after the intervention. At the KMC hospital’s clinical laboratory, blood samples were centrifuged before being kept overnight at −20°C in the biochemistry laboratory Human BDNF ELISA kit (YANNIC LIFE SCIENCES), Sandwich ELIZA kit for BDNF analysis. As instructed by the kit, ELIZA was carried out at 37°C. The biochemistry department at KMC Mangalore measured BDNF levels by reading the O.D. absorbance at 450 nm within 10 min after introducing the stop solution.[16]
Statistics
The Statistical Package for the Social Sciences version 25.0 was used to code and input the obtained data. A level of significance of <0.05 was assumed, with a 95% confidence interval.
The Chi-square test was used to compare baseline data such as gender, risk factors, stroke type, and participant hemiparesis side, and the Mann–Whitney U-test was used to compare variables such as age, post-stroke duration, BRS, MRS, and MMSE between groups. The Wilcoxon Signed-Rank test was used to compare the pre-and post-values of the groups’ STREAM, MBI, and BDNF values. Using the Mann–Whitney U-test, the median change scores of STREAM, MBI, and BDNF were compared between the groups.
RESULTS
The demographic baseline information for the various categories is shown in Table 1. Along with their p values, the median values for age, post-stroke time, BRS (UE, hand, and LE), MRS, and MMSE scores for both grouped and scattered individuals are provided. These findings imply that there are no appreciable changes between a MG and DG in terms of age, post-stroke duration, BRS (UE, hand, and LE), MRS, or MMSE scores. With p values of 0.252, 0.167, 0.704, and 0.767, respectively, the other demographic characteristics, such as gender, risk factors, stroke type, and side of hemiparesis, are equally distributed between the two groups.
| Variables | Massed group (n=16) | Distributed group (n=16) | P-value |
|---|---|---|---|
| Age* (years) | 62 [54.25–69.75] | 66 [53.5–77] | 0.669 |
| Gender+ | |||
| Men | 9 (56) | 13 (81.25) | 0.252 |
| Women | 7 (43.75) | 3 (18.75) | |
| Risk factors+ | |||
| Hypertension | 5 (31.25) | 5 (31.25) | 0.167 |
| Diabetes mellitus | 0 (0) | 3 (18.75) | |
| Hypertension and diabetes mellitus | 11 (68.75) | 6 (37.5) | |
| Others | 0 (0) | 2 (12.5) | |
| Type of stroke+ | |||
| Ischemic | 10 (62.5) | 12 (75) | 0.704 |
| Hemorrhagic | 6 (37.5) | 4 (25) | |
| Side of hemiparesis+ | |||
| Right | 9 (56) | 11 (68.75) | 0.716 |
| Left | 7 (43.75) | 5 (31.25) | |
| Post-stroke duration* (days) | 4.5 [2.25] | 4 [3–5] | 0.515 |
| BRS UE* | 3 [2–4] | 3 [2–5] | 0.897 |
| BRS Hand* | 3 [2–4] | 3 [2–4.75] | 0.897 |
| BRS LE* | 4 [2.25–5] | 3 [2–6] | 0.838 |
| MMSE* | 23.5 [23–26.5] | 23 [23–25] | 0.564 |
| MRS* | 4 [2.25–4] | 4 [3–5] | 0.468 |
The raw median of STREAM (UE, LE, and basic mobility), MBI, and BDNF for participants in both groups (massed and distributed) before and after intervention is shown in Table 2. Both massed and distributed practice groups showed post-intervention improvements in all three outcome measures (STREAM, MBI, and BDNF) with P = 0.05; however, the distributed practice group showed more pronounced changes than the massed practice group. When the groups' modified median STREAM (UE, LE, and basic mobility), MBI, and BDNF scores were compared, no statistically significant differences were seen with P > 0.05.
| Measure/group | Pre-intervention Median [IQR] | Post-intervention Median [IQR] | Within-group change Median [IQR] | Between-group change |
|---|---|---|---|---|
| STREAM UE | ||||
| MG | 10 [9.25–11.5] | 18 [10–20] | 3.5 [0.5–9.5]* | 0.110 |
| DG | 10 [2–10] | 13 [10–20] | 8 [6.25–9.75]** | |
| STREAM LE | ||||
| MG | 7.5 [6.25–10] | 14 [10–20] | 7 [3–10]** | 0.956 |
| DG | 7.5 [2–7.5] | 11.5 [7.5–19.5] | 6.5 [5–8]** | |
| STREAM basic mobility | ||||
| MG | 5 [4.25–13.75] | 17 [13–24] | 10 [6.5–11.5]** | 0.515 |
| DG | 5 [3–10] | 15.5 [8–21] | 8.5 [5–11.5]** | |
| STREAM total score | ||||
| MG | 23.5 [21–33.75] | 49.5 [33.5–63.75] | 19 [11.25–30]** | 0.838 |
| DG | 23.5 [7–32.5] | 41 [25.25–60.25] | 20 [18–28.75]** | |
| MBI | ||||
| MG | 19 [7.25–43] | 60.5 [26.5–83.25] | 26.5 [13.5–44.5]** | 0.323 |
| DG | 6.5 [0–17.5] | 45.5 [26.5–74.75] | 32 [25–48.75]** | |
| BDNF (ng/mL) | ||||
| MG | 0.65 [0.50–0.80] | 0.75 [0.625–0.875] | 0.05 [0.00–0.10]* | 0.419 |
| DG | 0.70 [0.60–0.80] | 0.80 [0.63–0.88] | 0.01 [0.001–0.15]* |
DISCUSSION
According to the findings of our study, both practice schedule groups (massed and distributed) demonstrated a discernible improvement across all outcome metrics. Animal studies have accumulated a growing body of evidence that suggests early exercise training (<72 h after a stroke) encourages neuroplastic changes, has positive effects on biomarkers, and improves functional recovery from stroke.[17,18] Few studies have focused on the importance of beginning an exercise-based physical rehabilitation intervention within the first 2 weeks following a stroke.[3,19] The key finding of the current study is that ASAP-based therapies improved learning due to neuroplastic changes, early physical therapy intervention in the window period, and may benefit very early stroke rehabilitation.
The key components of stroke therapy are recognized to be providing motor training at the right time and in the right amount.[20] All of the individuals in our study had active therapy for 45–60 min each day. As per the National Institute for Health and Care Excellence’s established guidelines for stroke rehabilitation, this might have aided the functional improvements of our participants.[21]
Motor learning, which is related to long-term changes in a patient’s capacity to perform a motor function after a stroke, leads to motor recovery.[22] Our ASAP-based therapy sessions included active participation, a problem-solving strategy that boosted their motivation and involved them in active participation to facilitate motivation, self-reliance, and self-determination, which may have sped up the learning process to improve stroke recovery.[23]
Enhanced STREAM scores, which are connected to motor learning, show that the repeated practice of the exercise prescribed by the therapist would have enhanced the quality of movement at the conclusion of therapy sessions.[24] The exercises given to the participants involved vigorous movement of the extremities in the controlled setting of the hospital, which offered the best learning environment.[10]
The massed practice group, which included stroke participants, engaged in the practice of physical activities in continuous and repeated training in a blocked way without clear start and stop points, which may have improved motor performances.[25] With greater BRSs and higher cognitive functioning (as measured by MMSE scores), the participants in this group may have recovered more quickly and effectively.
The distributed practice incorporates interval training for the person. According to this study, individuals in this group recovered more quickly than those in the mass practice group, showing that the spacing between activities in this group increased recovery rates and decreased participant’s fatigue.[26] Spacing decreased the training’s cognitive demand, resulting in greater post-rehabilitation recovery.[27] According to studies, spacing improves recollection of the task, the learning process, and ultimately results in greater motor skill performance.[28,29]
This follows a study by Partibhan et al. that demonstrated enhanced performance in a dispersed group where participant spacing reduced the cognitive strain.[30] Both practice groups’ BDNF levels increased post-intervention, although there were significant variations between massed practice and scattered practice. There is some weak evidence that suggests exercise training causes BDNF to be upregulated, which primes the brain to improve motor learning.[31] This rise in BDNF levels in our study may have aided participant performance and the learning process, resulting in improved motor function. In contrast to earlier research that used aerobic exercise and activity-dependent training, our study used ASAP rehabilitation and found that blood BDNF levels increased in sub-acute and chronic individuals.[32]
The intensity and frequency of exercises within sessions were raised during massed practice, which entailed continuous training without rest periods. In contrast, during distributed practice, the break periods caused the intensity of exercises within sessions to decrease. This can be related to the fact that BDNF values change significantly more during massed practice than during distributed practice because exercise conditions (such as type and intensity) control these changes.[33]
Limitation
The limitations of this study must be taken into account while analyzing the findings. First, the study evaluated the abilities that acute stroke subjects had learned following a 2-week intervention program. It was not possible to assess the retention of skills learned throughout the acute rehabilitation period due to shorter hospital stays. Therefore, a follow-up study that evaluates participant retention to determine the potential effectiveness of motor learning is required. Second, the study’s sample size was somewhat limited, making it uncertain whether the findings would apply to a larger population. Third, the early mobilization program’s Out of Bed activities lacked quantification. The qualitative measures were evaluated after the intervention, despite the possibility that this affected the reported outcomes. The monitoring of the intervention’s long-term effects was also limited due to its shorter duration. Future research should, however, take into account the elements impacting motor learning, such as external factors (feedback, task type, and environment) and internal factors (self-determination and self-efficacy).
Future line of research
The purpose of the research was to determine whether BDNF plays a significant role as a biomarker for assessing the degree of neural plasticity in strokes caused by obstruction of a small blood artery in the brain, which results in small artery diseases (SVD). On the functional Recovery and BDNF in SVD, additional impacts of massed and distributed practice can be seen.
CONCLUSION
This study focused on the skill-based training program in acute stroke rehabilitation by manipulating the practice sessions under the effect of ASAP’s core concepts. To the best of our knowledge, this study offers the first supporting data for early stroke rehabilitation using an ASAP-based intervention. In very early stroke therapy, distributed practice can be used to improve motor and functional recovery while also lowering cognitive and mental fatigue.
Ethical approval
The authors declare that they have taken the ethical approval from IEC, and the ethical approval number is IEC KMC MLR 11-18/418.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Global Health Statistics: A compendium of incidence, prevalence, and mortality estimates for over 200 conditions In: Global health statistics: A compendium of incidence, prevalence and mortality estimates for over 200 conditions. Geneva: World Health Organization; 1996. p. :7-906.
- [Google Scholar]
- Evidence behind stroke rehabilitation. J Neurol Neurosurg Psychiatry. 2003;74(Suppl 4):v18-21.
- [CrossRef] [PubMed] [Google Scholar]
- What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9:e87987.
- [CrossRef] [PubMed] [Google Scholar]
- Machine-based, self-guided home therapy for individuals with severe arm impairment after stroke: A randomized controlled trial. Neurorehabil Neural Repair. 2015;29:395-406.
- [CrossRef] [PubMed] [Google Scholar]
- Gains in upper extremity function after stroke via recovery or compensation: Potential differential effects on amount of real-world limb use. Top Stroke Rehabil. 2009;16:237-53.
- [CrossRef] [PubMed] [Google Scholar]
- Feasibility investigation of the Accelerated Skill Acquisition Program (ASAP): insights into reach-to-grasp coordination of individuals with post-acute stroke. Top Stroke Rehabil. 2013;20:151-60.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992-7.
- [CrossRef] [PubMed] [Google Scholar]
- Post-stroke BDNF foncentration changes following physical exercise: A systematic review. Front Neurol. 2018;9:637.
- [CrossRef] [PubMed] [Google Scholar]
- Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84-90.
- [CrossRef] [PubMed] [Google Scholar]
- Motor learning and control: Concepts and applications (9th ed). New York: McGraw Hill; 2011.
- [Google Scholar]
- Association of activity changes in the primary sensory cortex with successful motor rehabilitation of the hand following stroke. Neurorehabil Neural Repair. 2012;26:881-8.
- [CrossRef] [PubMed] [Google Scholar]
- Normalized Mini-Mental State Examination for assessing cognitive change in population-based brain aging studies. Neuroepidemiology. 2014;43:15-25.
- [CrossRef] [PubMed] [Google Scholar]
- Infusing motor learning research into neurorehabilitation practice: A historical perspective with case exemplar from the accelerated skill acquisition program. J Neurol Phys Ther. 2014;38:190-200.
- [CrossRef] [PubMed] [Google Scholar]
- Inter-rater reliability and validity of the stroke rehabilitation assessment of movement (stream) instrument. J Rehabil Med. 2002;34:20-4.
- [CrossRef] [PubMed] [Google Scholar]
- Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol. 2017;17:131.
- [CrossRef] [PubMed] [Google Scholar]
- Proprioceptive neuromuscular facilitation (PNF) vs. task-specific training in acute stroke: The effects on neuroplasticity. MOJ Anat Physiol. 2018;5:154-8.
- [CrossRef] [Google Scholar]
- Neuroprotection of early locomotor exercise poststroke: Evidence from animal studies. Can J Neurol Sci. 2015;42:213-20.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: A rat brain ischemia model. PLoS One. 2011;6:e16643.
- [CrossRef] [PubMed] [Google Scholar]
- Early rehabilitation after stroke: A narrative review. Curr Atheroscler Rep. 2017;19:59.
- [CrossRef] [PubMed] [Google Scholar]
- Dose and timing in neurorehabilitation: Prescribing motor therapy after stroke. Curr Opin Neurol. 2015;28:549-55.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke rehabilitation: Therapy. 2020. :1-15. Available from: https://pathways.nice.org.uk/pathways/stroke [Last accessed on 2020 Apr 20]
- [Google Scholar]
- Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197-205.
- [CrossRef] [PubMed] [Google Scholar]
- Brain plasticity and recovery from early cortical injury. Dev Psychobiol. 2007;49:107-18.
- [CrossRef] [PubMed] [Google Scholar]
- Motor learning principles for neurorehabilitation. Handb Clin Neurol. 2013;110:93-103.
- [CrossRef] [PubMed] [Google Scholar]
- A review of “The co-ordination and regulation of movements” by N. Bernstein (Pergamon press, 1967) Ergonomics. 1968;11:95-7.
- [CrossRef] [Google Scholar]
- Comparing the effect of massed and distributed practice in different stages of discrete motor task learning. Sport Sci. 2011;1:101-6.
- [Google Scholar]
- Inflammatory signaling in post-stroke fatigue and depression. Eur Neurol. 2018;80:138-48.
- [CrossRef] [PubMed] [Google Scholar]
- Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull. 2006;132:354-80.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of massed practice versus distributed practice on basic mobility skills among post-stroke patients. Int J Physiother Res. 2019;7:3028-3.
- [CrossRef] [Google Scholar]
- Promoting neuroplasticity for motor rehabilitation after stroke: Considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93:1707-16.
- [CrossRef] [PubMed] [Google Scholar]
- Increased serum brain-derived neurotrophic factor with high-intensity interval training in stroke patients: A randomized controlled trial. Ann Phys Rehabil Med. 2020;64:101385.
- [CrossRef] [PubMed] [Google Scholar]
- Gradually increased training intensity benefits rehabilitation outcome after stroke by BDNF upregulation and stress suppression. Biomed Res Int. 2014;2014:925762.
- [CrossRef] [PubMed] [Google Scholar]







