Translate this page into:
Visual Aura in Migraine: An Analysis of 165 Patients in a Tertiary Care Hospital in North India
Monika Singla, DM (Neurology) 20-A, Rajguru Nagar, Near MBD Mall, Ludhiana, 141012, Punjab India drmonika78@yahoo.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Migraine auras are transient neurological symptoms, usually lasting for approximately 5 to 30 minutes before the onset of migraine pain. Out of various types of auras, visual aura is the commonest and has variable manifestations, forming approximately 90% of auras. These visual auras may be of particular interest to the ophthalmologist as well as to the neurologist. We planned to conduct this study to look for the prevalence of visual aura in our population and make a descriptive analysis of the same.
Materials and Methods It was an observational, questionnaire-based cross-sectional study, enrolling all the consenting patients of migraine. Migraine was classified by International Classification of Headache Disorders (ICHD)-III β version, Third edition of International Classification of Headache Disorders. Patients in whom aura was present, detailed profile of visual aura was made regarding its type, duration, relation with migraine as per its laterality, etc.
Observations and Results Out of 1,245 migraine patients, 165 (13.25%) patients reported to have visual aura, 127 females and 38 males. Scintillating scotoma was the commonest type of visual aura, then zigzag lines, blurred vision, and tunnel vision. Majority of patients had aura between 5 and 35 minutes, none had more than 60 minutes. A total of 142 patients out of 165 had unilateral aura, out of which 64 (38.78%) patients had aura ipsilateral to the side of headache, and 78 (47.27%) patients had aura contralateral to the side of headache. Twenty-three (13.93%) patients had bilateral aura.
Discussion The frequency of visual aura was found to be 13.25% in our study, which is high compared with previously published Indian data. We did a descriptive analysis of visual aura symptoms.
Conclusion Visual aura is the commonest type of aura, more frequent in females. Scintillating scotoma was found to be the commonest type of visual aura, followed by zigzag lines in study. Our study is unique of its type as its shows a descriptive visual analysis in a larger number of patients.
Keywords
migraine with aura
visual aura
scintillating scotoma
transient ischemic attack
Introduction
Migraine is a clinical syndrome of unknown etiology, leading to a variety of visual disturbances. Migraine with aura is an incapacitating myriad of transient neurological symptoms, often characterized by aura disturbances which can be visual and occasional sensory or dysphasic, preceding headache. Prevalence of migraine is variable as per different studies; it is 8% in general population as reported by Russel et al.1 Amongst all forms of aura disturbances the frequency of visual aura cases (98–99%) is far more than sensory (36%) and language (10%) dysfunctions.2 In another study, migraine with typical visual aura was reported in more than 30% of their attacks, white spots, zigzag lines, flashing lights, and scotoma being the most frequently prevalent visual aura symptoms.3 Though visual aura symptoms are complex visual disturbances and mostly multifaceted, they have a marked heterogeneity without any pathophysiological explanation.4 The main characteristics of visual aura may include exclusively visual aura with and without headache, acute onset (sudden) visual aura, sensory and motor auras, unilateral or bilateral visual auras followed by unilateral or bilateral headache. A typical visual aura can initiate as a flickering, unilateral, uncolored squiggly line. It may start in the center with gradual spread toward the periphery.5 The progression of visual aura across the visual field is slow, i.e., >4 minutes, with a gradual return to normalcy within 20 minutes to an hour, contrary to cerebral ischemia which is usually sudden in onset. In contrast to seizures the progression from positive phenomenon (zigzag vision, shimmering, tingling, or scintillations) to negative symptoms (scotoma) is paced and gradual in migraine with aura and sometimes in form of complex infrequent perceptions like tunnel vision, hallucinations, etc.6 As per International Classification of Headache Disorders (ICHD)-3 β version, the diagnostic criteria include at least two attacks with one or more fully reversible aura symptoms like visual, speech, sensory, brainstem, motor, or retinal, with gradual spread over ≥5 minutes, lasting between 5 and 60 minutes, occurring in succession. One aura symptom may be unilateral, the aura is usually followed by headache mostly on the opposite side.7 8 The marked inter and intrapatient variability amongst various forms of aura suggests varied and distinct underlying anatomical dysfunctions.9 Migraine visual aura can be seen independently without headache in approximately 3% of the general population.10 Visual disturbances of migraine generally last less than an hour, but sometimes may last for days to even months.11 12 As per literature, migraine with visual aura has increased risk of vascular events like stroke, transient ischemic attacks, myocardial infarction, atrial fibrillation as compared with patients of migraine without aura.13 14 15
Despite being thoroughly studied, descriptive analysis of visual aura with migraine has not been reported much. Therefore, we planned to conduct a study in our patients of migraine to study the presence of visual aura and make a comprehensive list of all the visual symptoms present in them.
Methods
The study was conducted in a tertiary care hospital, the South-Western corner of Punjab, in North India. This hospital mainly caters to rural population. We screened all the patients who presented to our neurology outpatient department with complaints of headache. All patients who consented to take part and fulfilled ICHD-III β version and 2013 criteria for migraine were enrolled in the study. Study was conducted between August 2016 and January 2018. Institutional ethics clearance was taken for the same. Patients with one-time headache, secondary headaches, and tension headache were excluded from the study. We could not identify any patient with trigeminal autonomic cephalgias. Out of 1,586 patients, 1,245 patients of migraine were included in the study, out of which 937 were female patients and 308 were males. A structured questionnaire was applied to all the participants. A medical student was trained for the same; he was also trained regarding questionnaire related with aura and types of aura. Questionnaire included four sections, first section was related with demographic factors including age, sex, marital status, occupation, and education. Another section dealt with questions related with first contact of patient regarding diagnosis, who diagnosed their first headache, a general physician, a neurologist, other or they did not contact anyone? Third section had questions related with severity of headache, description and location of headache, along with accompanying features like photophobia, phonophobia, nausea, vomiting, numbness, ringing sensation, dizziness etc. Then in the last section, questions regarding light sensitivity, triggers of migraine, and various co-morbidities were asked. MIDAS score was also applied for all patients to assess migraine-related disability. A necessary clinical examination was performed on all the patients. To all the patients of migraine, detailed questions related with aura, types of aura, duration of aura were asked. For visual aura, a questionnaire was applied regarding type of visual symptoms, positive or negative symptoms. Patients were enquired regarding duration of headache, relation of aura with headache episode, number and types of auras or overlap of auras if they had. Patients were requested to draw the aura on a paper from their memory which they experienced during the attacks. In case of any doubt about aura symptoms, it was confirmed by the treating neurologist. Clinical examination was performed by the neurologist on all the patients, especially in patients with migraine with aura for any focal deficits. Fundus examination was performed by ophthalmologist in all the patients with visual aura, especially with scotomas. Computed tomography of head was performed in all the patients with visual aura. Magnetic resonance imaging (MRI) of brain was done in selected patients where there were doubtful symptoms. Though MRI brain with magnetic resonance angiography is preferred in migraine with aura attacks, it was not possible in our hospital as there was huge rush in hospital for neuroimaging of the brain so it was not possible to get MRI brain on the same day in most of the patients.
Statistical Analysis
SPSS Statistics v25, SAS Studio 3.7 and Excel (Office 365) were primarily used for analyzing data. Exploratory data analysis was performed on explanatory variables (such as demographic variables). Distribution analysis was conducted for independent variables and the data was visualized using flowcharts, tables, and Venn diagrams.
Observations and Results
Out of 1,586 patients of headache, 1,518 consented to take part in the study. After excluding 273 nonmigraine headache patients, 1,245 patients were enrolled for the study. Out of 1,245 patients, 180 (14.46%) patients reported to have aura (Fig. 1 , flowchart). 14.46% patients had migraine with aura, with visual aura being the commonest out of all auras with frequency of 13.25%. Therefore 165 (91.66% of all auras) patients reported to have visual aura, 127 being female patients and 38 males. Baseline characteristics of patients of migraine with aura and migraine without visual aura have been described in detail in Table 1. In both the groups, females outnumbered males and the most common affected age group was 25 to 44 years in both the groups. On comparing both the groups, migraine without visual aura and migraine with visual aura, there was no statistical difference in terms of gender, age range, marital status, education, type of pain, pain intensity in both the groups. Mean age of migraine without aura group was 36.66 years (±13.42), with confidence interval of 35.85 to 37.47 and p < 0.001 and in migraine with aura group 37.91 years (±13.31), with confidence interval of 35.85 to 39.98 and p < 0.001. Age being exposure variable shows significant impact on disease outcome in both the groups. Regarding duration of pain attack, 1,106 (88.83%) patients had attacks lasting less than 24 hours. Only 52 patients (4.17%) had pain lasting for more than 24 hours, out of these, six patients were found to have medication overuse related to headache as they were consuming regular analgesics. The number of headache attacks varied from two to 17 per month in migraine without aura group and from one to five attacks in migraine with aura group. On comparing comorbidities, depression and syncope were found more commonly in migraine with aura group and it was statistically significant (p < 0.05), though anxiety was found more in migraine without aura group (p < 0.05).
|
Migraine without visual aura |
Female N (%) |
Male N (%) |
Migraine with visual aura |
Female N (%) |
Male N (%) |
||
|---|---|---|---|---|---|---|---|
|
Total N (%) |
Total N (%) |
||||||
|
Gender |
1,065 (100%) |
799 (75.02%) |
266 (24.98%) |
165 (100%) |
127 (76.97%) |
38 (23.03%) |
0.589 |
|
Age vitals |
Mean age |
Mean age |
|||||
|
36.66 y (±13.42) p <0.001 |
37.91 y (±13.31), p <0.001 |
||||||
|
Total N (%) |
Female N (%) |
Male N (%) |
Total N (%) |
Female N (%) |
Male N (%) |
||
|
Age |
|||||||
|
Up to 24 y |
187 (17.56%) |
112 (59.89%) |
75 (40.11%) |
21 (12.73%) |
14 (66.67%) |
7 (33.33%) |
0.246 |
|
25–44 y |
549 (51.55%) |
437 (79.60%) |
112 (20.40%) |
95 (57.58%) |
76 (80.00%) |
19 (20.00%) |
|
|
45–64 y |
287 (26.95%) |
227 (79.09%) |
60 (20.91%) |
40 (24.24%) |
31 (77.50%) |
9 (22.50%) |
|
|
>65 y |
42 (3.94%) |
23 (54.76%) |
19 (45.24%) |
9 (5.45%) |
6 (66.67%) |
3 (33.33%) |
|
|
Marital status |
(0.00%) |
||||||
|
Married |
896 (84.13%) |
707 (78.91%) |
189 (21.09%) |
143 (86.67%) |
114 (79.72%) |
29 (20.28%) |
0.403 |
|
Unmarried |
169 (15.87%) |
92 (54.44%) |
77 (45.56%) |
22 (13.33%) |
13 (59.09%) |
9 (40.91%) |
|
|
Education |
|||||||
|
Undergraduates |
819 (76.90%) |
588 (71.79%) |
231 (28.21%) |
133 (80.61%) |
99 (74.44%) |
34 (25.56%) |
0.171 |
|
Post-graduation |
54 (5.07%) |
46 (85.19%) |
8 (14.81%) |
3 (1.82%) |
3 (100.00%) |
0 (0.00%) |
|
|
Illiterate |
192 (18.03%) |
165 (85.94%) |
27 (14.06%) |
29 (17.58%) |
25 (86.21%) |
4 (13.79%) |
|
|
Pain type |
Total instances 1,314 |
Total Instances 209 |
|||||
|
Aching |
280 (21.31%) |
200 (71.43%) |
80 (28.57%) |
43 (20.57%) |
29 (67.44%) |
14 (32.56%) |
0.950 |
|
Throbbing/pulsating |
280 (21.31%) |
217 (77.50%) |
63 (22.50%) |
40 (19.14%) |
34 (85.00%) |
6 (15.00%) |
0.576 |
|
Sharp/lancing |
446 (33.94%) |
332 (74.44%) |
114 (25.56%) |
67 (32.06%) |
55 (82.09%) |
12 (17.91%) |
0.757 |
|
Pressure/squeeze |
308 (23.44%) |
231 (75.00%) |
77 (25.00%) |
59 (28.23%) |
46 (77.97%) |
13 (22.03%) |
0.741 |
|
Pain intensity in last 12 mo |
Total instances 1,080 |
Total Instances 165 |
|||||
|
Mild |
130 (12.04%) |
85 (65.38%) |
45 (34.62%) |
13 (07.88%) |
5 (38.46%) |
8 (61.54%) |
0.261 |
|
Moderate |
338 (31.30%) |
240 (71.01%) |
98 (28.99%) |
51 (30.91%) |
36 (70.59%) |
15 (29.41%) |
|
|
Severe |
612 (56.67%) |
484 (79.08%) |
128 (20.92%) |
101 (61.21%) |
86 (85.15%) |
15 (14.85%) |
|
|
Comorbidities |
Total instances 287 |
Total Instances 72 |
|||||
|
Depression |
49 (17.07%) |
31 (63.27%) |
18 (36.73%) |
18 (25%) |
12 (85.71%) |
2 (14.29%) |
0.0008 |
|
Syncope |
63 (21.95%) |
42 (66.67%) |
21 (33.33%) |
21 (29.17%) |
10 (66.67%) |
5 (33.33%) |
0.001 |
|
Anxiety |
160 (55.75%) |
116 (72.50%) |
44 (27.50%) |
44 (61.11%) |
23 (63.89%) |
13 (36.11%) |
0.001 |
|
Others |
15 (5.23%) |
12 (80.00%) |
3 (20.00%) |
3 (4.16%) |
5 (71.43%) |
2 (28.57%) |
0.683 |
|
Medications |
|||||||
|
Beta blockers |
140 (13.14) |
84 (10.51%) |
56 (21.05%) |
65 (39.39%) |
43 (33.85%) |
22 (57.89%) |
|
|
Tricyclic antidepressants |
88 (8.26%) |
53 (6.63%) |
35 (13.16%) |
39 (23.63%) |
35 (27.56%) |
4 (10.53%) |
|
|
Topiramate |
35 (3.28) |
30 (3.75%) |
5 (1.88%) |
13 (7.88) |
10 (7.87%) |
3 (7.89%) |
|
|
Flunarizine |
33 (31%) |
23 (2.87%) |
10 (3.76%) |
9 (5.45%) |
8 (6.3%) |
2 (5.26%) |
|
|
Analgesics |
110 (1 0.33%) |
88 (11.01%) |
22 (8.27%) |
12 (7.27%) |
9 (7.09%) |
2 (5.26%) |
|
|
No treatment |
659 (61.88%) |
521 (65.2%) |
138 (51.87%) |
27 (16.36%) |
22 (17.32%) |
5 (13.16%) |

-
Fig. 1 Patient flowchart.
Fig. 1 Patient flowchart.
We applied MIDAS questionnaire to all patients with migraine with aura to access the disease-related disability and its impact on their quality of lives in the previous 3 months. About 87 patients (52.73%) had mild disability, 23 patients (13.93) had little or no disability, 33 patients (20%) had moderate disability, and 22 patients (13.33%) had severe disability. Most common accompanying feature found in our migraine with visual aura patients was fatigability, found in 77 patients (46.67%), followed by nausea/vomiting in 52 patients (31.52%), then photophobia in 50 patients (30.3%), numbness, vertiginous sensation, and tingling in the remaining patients. Common trigger factors for migraine attacks were stress, noise, exertion, sleep disturbances, smell, and hormonal factors.
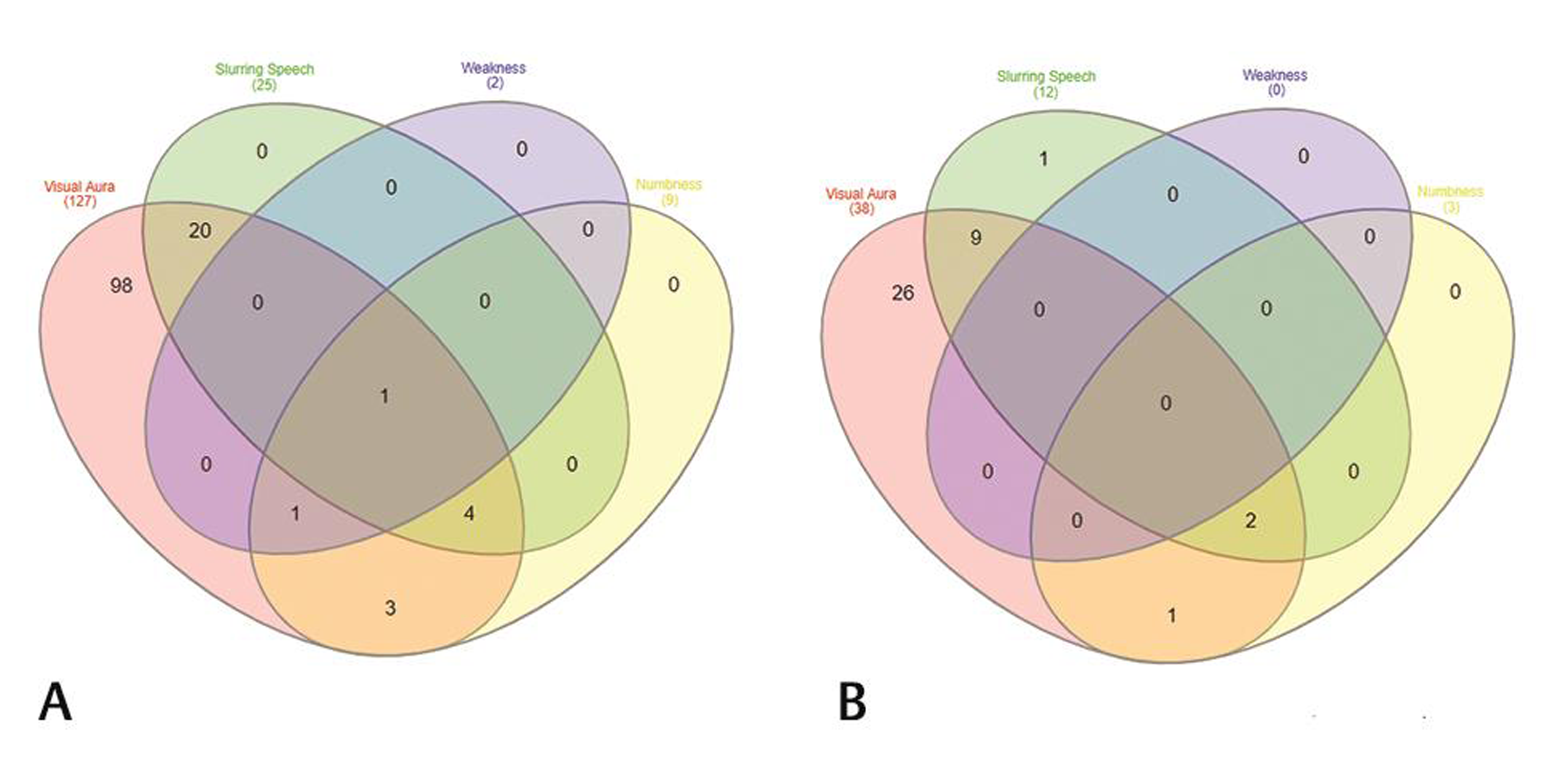
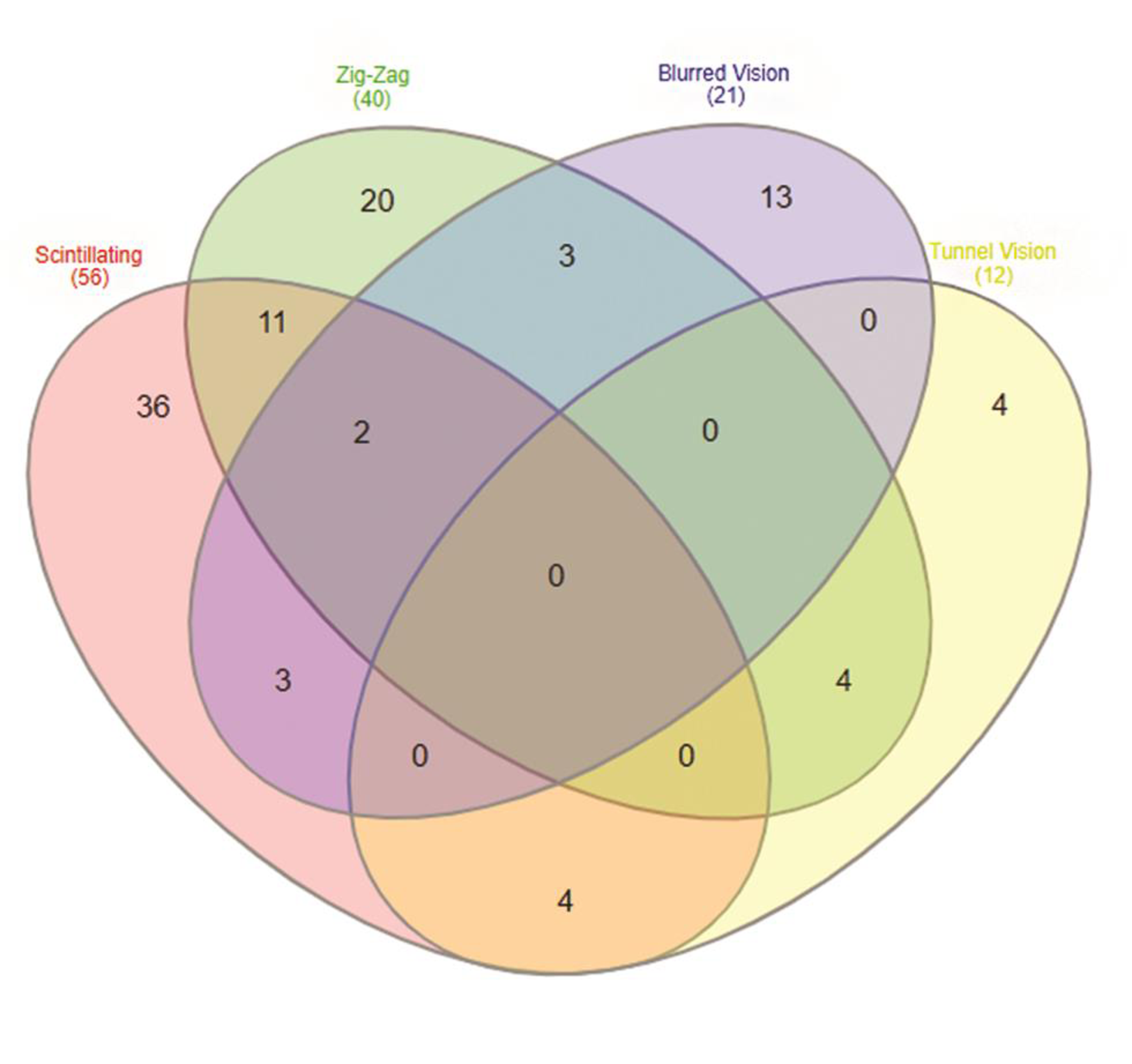
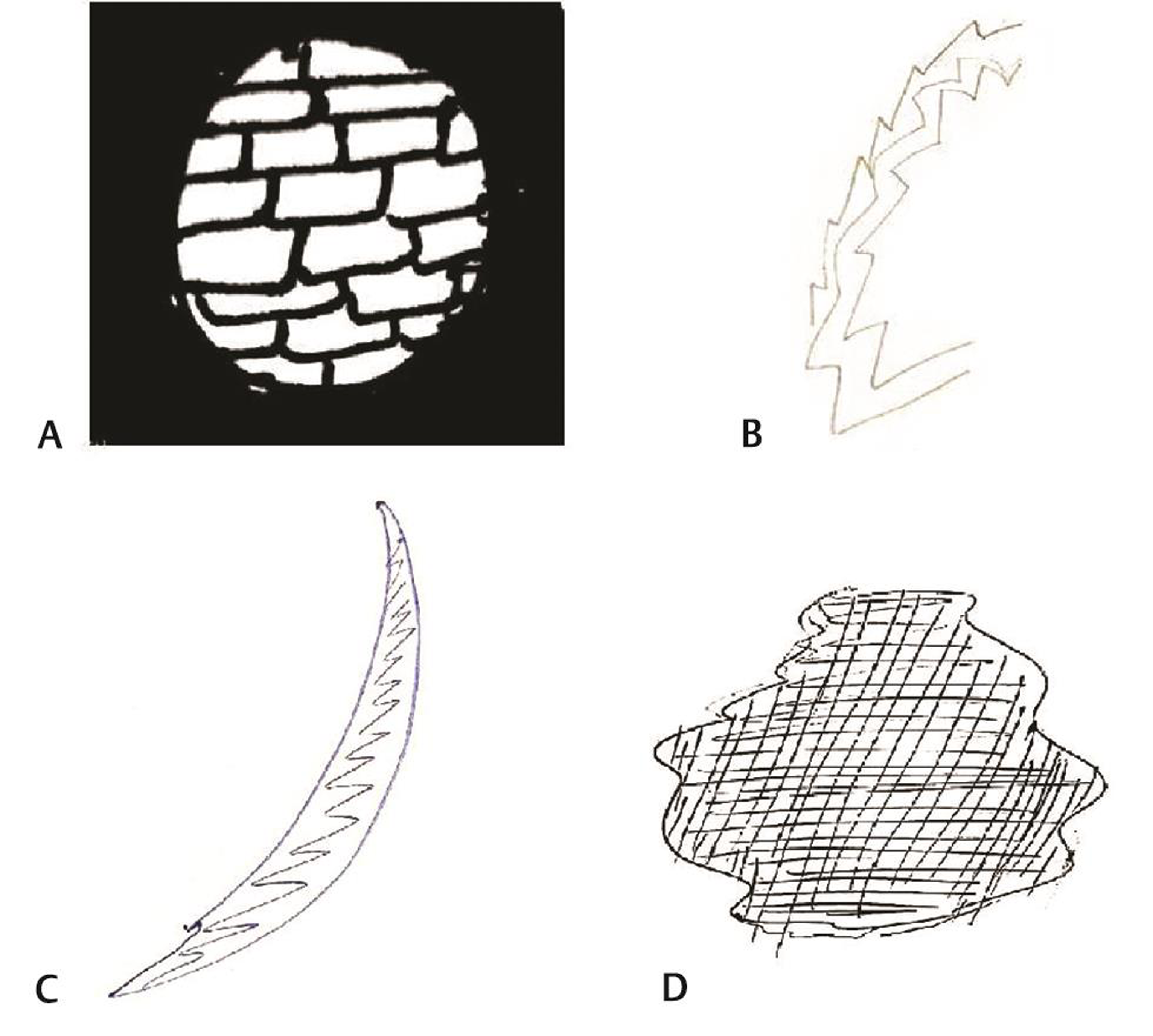
On analyzing the types of auras, 165 patients were found to have visual aura, 37 patients (20.5%) complained of slurring of the speech preceding headache, numbness as aura was seen in 12 (6.7%) patients, and weakness (hemiplegia) was seen in only two patients (1.1%) (Table 2). Venn diagram (Fig. 2A, B) shows overlap of various types of auras in females and males. On doing gender wise comparison amongst aura types, there was no statistically significant difference found in both the genders with regard to various aura types. A total of 105 (63.63%) patients had aura preceding headache, 24 (14.54%) patients had headache preceding aura. Patients with visual aura did not have headaches during all the episodes, 36 (21.8%) patients were found to have only aura without any headache. The duration of aura was variable between 5 and 35 minutes. No patient had any persistent aura. One hundred and forty-two patients had unilateral aura, out of which 64 (38.78%) patients had aura ipsilateral to the side of headache, 78 (47.27%) patients had aura contralateral to the side of headache. Twenty-three (13.93%) patients had bilateral aura (Table 3). Twenty-one percent of the patients had more than one type of aura and 5% had even two or three types of aura. Amongst the patients with visual aura also, 23% of the patients had overlap of various types of auras. Venn diagram shows overlap of four most common visual auras (Fig. 3). Scintillating scotoma was the commonest type of aura present in 39.39% of the patients; next common was zigzag lines and blurring of vision. Details of various types of auras are mentioned in Table 4. Fig. 4 (A–D) shows illustrations of visual aura of four patients felt during the migraine attack and drawn on a paper during their outdoor patient department visits.
|
Aura type |
Total N (%) |
Female N (%) |
Male N (%) |
p-Value |
|---|---|---|---|---|
|
Visual aura |
165 (100%) |
127 (76.97%) |
38 (23.03%) |
0.671 |
|
Slurring of speech |
37 (22.42%) |
25 (67.57%) |
12 (32.43%) |
|
|
Weakness |
2 (1.21%) |
2 (100.00% |
0 (0.00%) |
|
|
Numbness |
12 (7.27%) |
9 (75.00%) |
3 (25.00%) |
|
Number of patients |
Percentage |
|
|---|---|---|
|
Number of visual aura |
||
|
Females |
127 |
76.97 |
|
Males |
38 |
23.03 |
|
Duration of aura |
||
|
5–30 min |
105 |
63.63 |
|
31–60 min |
60 |
36.36 |
|
60 min |
0 |
0 |
|
Unilateral aura |
||
|
Ipsilateral |
64 |
38.78 |
|
Contralateral |
78 |
47.27 |
|
Bilateral aura |
23 |
13.93 |
|
Aura preceding headache |
105 |
63.63 |
|
Aura without headache |
36 |
21.8 |
|
Headache preceding aura |
24 |
14.54 |
|
Number of patients had headache episodes without aura |
23 |
13.93 |
|
Number of patients |
Percentage |
|
|---|---|---|
|
Scintillating scotoma |
56 |
33.93 |
|
Zigzag lines |
40 |
24.24 |
|
Blurred vision |
21 |
12.72 |
|
Tunnel vision |
12 |
7.27 |
|
Flashes of bright light |
19 |
11.51 |
|
Blind spots |
19 |
1.51 |
|
Macropsia |
14 |
8.48 |
|
Micropsia |
13 |
7.87 |
|
Complex hallucinations |
5 |
3.03 |
|
Flickering lights |
15 |
9.09 |
|
Visual snow |
7 |
4.24 |
|
Things look farther than they are |
9 |
5.45 |
|
White spots |
5 |
3.03 |
|
Hemianopsia |
3 |
1.81 |
|
Like a mosaic |
2 |
1.21 |
|
Crescent shaped |
2 |
1.21 |
|
Colored lines |
1 |
0.60 |
-
Fig. 2 (A) Venn diagram showing overlap of four aura types found in our population, visual aura, slurring of speech, weakness, and numbness in female patients and (B) in male patients.
Fig. 2 (A) Venn diagram showing overlap of four aura types found in our population, visual aura, slurring of speech, weakness, and numbness in female patients and (B) in male patients.
-
Fig. 3 Venn diagram showing overlap of four commonest types of visual auras found in our study population, i.e., scintillating scotoma, zigzag lines, blurred vision, and tunnel vision.
Fig. 3 Venn diagram showing overlap of four commonest types of visual auras found in our study population, i.e., scintillating scotoma, zigzag lines, blurred vision, and tunnel vision.
-
Fig. 4 (A) Case Vignette 1—picture depicting aura of a patient which he experienced while he was at his construction site. He started seeing only part of the wall in the center and as if other part of wall disappeared suggestive of tunnel vision followed by headache. (B) Case Vignette 2—picture depicting aura of a patient while working in her office, felt wavy lines suggestive of zigzag aura. (C) Case Vignette 3—suggestive of scintillating scotoma experienced by a patient preceding a migraine attack as visual aura while she was teaching her students, she could not see her class properly, she was seeing moving shining arc-shaped scotoma. (D) Case Vignette 4—a 35-year-old female suffered from an episode while she was teaching her students in school. She started seeing a black spot in her vision, which lasted for few minutes, followed by severe unilateral pulsatile headache. It lasted for about 5 hours and then settled down.
Fig. 4 (A) Case Vignette 1—picture depicting aura of a patient which he experienced while he was at his construction site. He started seeing only part of the wall in the center and as if other part of wall disappeared suggestive of tunnel vision followed by headache. (B) Case Vignette 2—picture depicting aura of a patient while working in her office, felt wavy lines suggestive of zigzag aura. (C) Case Vignette 3—suggestive of scintillating scotoma experienced by a patient preceding a migraine attack as visual aura while she was teaching her students, she could not see her class properly, she was seeing moving shining arc-shaped scotoma. (D) Case Vignette 4—a 35-year-old female suffered from an episode while she was teaching her students in school. She started seeing a black spot in her vision, which lasted for few minutes, followed by severe unilateral pulsatile headache. It lasted for about 5 hours and then settled down.
Discussion
Our study evaluated the characteristics of aura in migraine in detail. All patients were diagnosed with ICHD-III β version 2013 criteria. All patients underwent a structured questionnaire including multiple sections pertaining to migraine characteristics and migraine with aura. Our study was conducted over a large sample of patients. Ours was a hospital-based cross-sectional study; patients were questioned regarding their aura experience. In our study frequency of visual aura was found to be 13.25% which is high compared with previously reported Indian studies.16 The explanation for the high prevalence may be that our study was conducted in a tertiary care hospital and patients with aura symptoms are more likely to report to the hospital, considering it to be serious. In our study visual aura was found to be the commonest. Our findings were comparable to the study narrated by Evans, where visual aura (99%) outnumbered sensory (54%) and aphasic (32%) auras.6
Twenty-four (14.54%) patients had headache preceding aura and 36 (21.8%) patients were found to have only aura without any headache in our study. Majority of the aura patients, i.e., 63.63% patients had aura preceding headache, which is comparable to data reported by Queiroz et al17 in 57%, Kelman18 in 67.4%, and Russell and Olesen5 in 92%, had aura exclusively before headache. The duration of the visual auras in our study was 5 to 30 minutes seen in 63.63% of the patients which is quite similar to findings reported by Queiroz et al17 (59%), Bana and Graham19 (64.7%), Kelman18 (63%), Russell and Olesen5 (69%), and Manzoni et al20 (75.6%), Cologno et al21 (65.4%) where the reported duration of aura was 5 to 30 minutes.
In our study, only 23 (13.93%) patients had bilateral aura. Unilateral auras are reported in majority, i.e., 142 patients. This finding is also comparable with the study done by Queiroz et al22 as they reported in unilaterality in 76 patients out of 122 patients.
Twenty-one percent of the patients had more than one type of aura and 5% had even two or three types of aura. Amongst the patients with visual aura also, 23% of the patients had overlap of various types of auras. Scintillating scotoma was the commonest type of aura present in 33.93% of the patients; next common was zigzag lines and blurring of vision. Queiroz et al22 reported “blurred/foggy vision” as the most common symptom and “small bright dots” as the second most frequent visual hallucination in their study. This is similar to Queiroz et al17 as they found this in 42% of the patients. Eriksen et al23 reported zigzag lines in 58% of the patients and Russell and Olesen5 patients reported these lines in 81% of the patients. We compared our findings with previously published data in view of types of aura, gender preference, laterality, and colored/uncolored hallucinations24 (Table 5).
|
Reference no. |
Author |
Year |
Study setting |
Study type |
Patient number |
Gender distribution male/female |
Frequency of different auras nonvisual/visual aura |
Common visual aura symptoms |
||
|---|---|---|---|---|---|---|---|---|---|---|
|
Present study |
Singla et al. |
2020 |
One center |
Prospective, cross-sectional study |
180/1,245 |
23.03% |
76.97% |
Slurring of speech (22.42%), weakness (1.21%), numbness (7.27%) out of 180 |
165 of 180 |
Scintillating scotoma (33.93%) Zigzag lines (24.24%) Blurred vision (12.74%) Tunnel vision (7.27%). |
|
3 |
Hansen et al. |
2016 |
16 centers in the United States |
Double-blind, placebo-controlled trial |
267 |
19% |
81% |
52% |
17.60% |
Dots or flashing lights, wavy or jagged lines, blind spots, and tunnel vision. |
|
4 |
Viana et al. |
2019 |
14 publications included |
Systematic review |
Flashes of bright light (16–38%), foggy vision (25–54%), zigzag lines (24–81%), scotoma (23–77%), flickering light (12–91%). |
|||||
|
5 |
Russell and Olesen |
1996 |
National Central Population Registry, Denmark |
Questionnaire-based retrospective study |
4,000 (163 had migraine with aura) |
75% |
25% |
Sensory (31%), aphasic (18%) and motor (6%) |
161 of 163 patients (99%) |
Flickering light of various colors, uncolored zigzag lines (fortification), scotoma |
|
6 |
Evans |
2014 |
Critical appraisal of various studies |
362 |
Sensory aura (54%), aphasic aura (32%) |
99% |
Blurred vision, 54.1%; small, bright dots, 47.5%; zigzag lines, 41.8%; flashes of bright light, 38.5%; blind spots, 33.6%; flickering light, 30.3%; like looking through heat waves or water, 24.6%; colored dots/spots of light, 19.7%; and tunnel vision, 9.8%. |
|||
|
18 |
Kelman |
2004 |
One center in the United States |
Review of a clinical database |
952 (38% had aura) |
27.50% |
36.80% |
Speech difficulties (17.3%) |
60.80% |
No mention |
|
22 |
Queiroz et al. |
2011 |
Two centers (Southern Brazil and Northern United States) |
Retrospective, descriptive study |
122 |
16.39% |
83.60% |
Not mentioned |
82.80% |
Blurred vision (54.1%), small bright dots (47.5%), zigzag lines (41.8%) |
|
23 |
Eriksen et al. |
2004 |
Danish patient registry and from various Danish neurology practices |
Semistructured physician-conducted interviews |
362 |
27.34% |
72.65% |
Sensory (54%), aphasic (32%) |
99% |
Flickering light, scotoma, zigzag lines |
|
24 |
Eriksen et al. |
2005 |
Danish patient registry and from various Danish neurology practices |
Semistructured physician-conducted interviews |
427 |
26.46% |
73.54% |
25.29% |
74.71% |
Zigzag lines (fortification), flickering light, scotoma |
Regarding the pathophysiology and blood flow studies related to laterality of aura and headache, Olesen et al25 had studied headache and aura laterality. They studied regional cerebral blood flow (rCBF) and found information about laterality. They have reported that rCBF changes are virtually unilateral. In areas of low blood flow, there is origin of aura symptoms. Abnormalities in rCBF unilaterally were significantly associated with ipsilateral or bilateral headache. Aura that is usually unilateral is significantly associated with contralateral or bilateral headache. Regarding laterality of aura and rCBF abnormalities, they reported these to be contralateral to each other.
We planned to do the comprehensive analysis of visual aura in our study population. Migraine aura itself is a risk factor for cerebrovascular diseases like stroke and heart diseases including myocardial infarction and atrial fibrillation.14 26 27 Moreover migraine is more common in females in young age and use of oral contraceptives leads to 13 times increased risk of stroke and thromboembolism in patients with migraine with aura.28 29 Therefore it is very important to understand aura and its types in a better way. This will help in planning future studies. Aura usually has gradual onset suggestive of cortical spreading depression as the pathophysiology, therefore it can be differentiated from ischemia, inflammation, and compression.29 30 Panayiotopoulos has stated regarding differentiating occipital seizures from visual auras on the basis of color of visual hallucinations. Auras in seizures are usually circular/spherical in forms and multicolored, and the auras of migraine are black-and-white, with linear/zigzag lines.31 32 Russel et al reported colored auras in 13 attacks (24%) out of 54 aura attacks in 20 patients.33 Therefore, it is very important to correctly diagnose these visual symptoms as aura and differentiate them from transient ischemic attacks, stroke, and seizure. Our study has this limitation as we did not ask regarding colors of auras and family history. We also did not do MRI of the brain with magnetic resonance angiography in our patients during attacks of aura to differentiate from stroke or transient ischemic attack. Another limitation of our study was that patient’s responses were not recorded during the aura attacks; they responded to our questions from their memory, so there can be a recall bias in our study.
Our study is unique in some aspects that we conducted this observational, cross-sectional study on a larger population and we could find a large number of patients with visual aura in our population as compared with previously published studies. Then we tried to explain the various clinical aspects of visual aura in detail. This kind of descriptive analysis of visual aura was not performed frequently. As migraine with aura is much more common in females as compared with males, awareness can be created in females of reproductive age group regarding the use of oral contraceptives and its increased risk associated with stroke.
Conclusion
Visual aura was found to be the commonest in our study, in 13.25% of the patients. We gave a descriptive analysis of various types of visual aura in our study. Visual auras were heterogenous in our study. The maximum duration of aura was found to be 35 minutes. Most of the auras were unilateral. There was no fixed relationship with headache duration and side of headache with aura. Out of various types of aura, scintillating scotoma, zigzag lines, tunnel vision, and blurred vision were found to be the commonest in our study.
Note
The study primarily carried out at Guru Gobind Singh Medical College, Faridkot
Conflict of Interest
None declared.
References
- Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol. 1995;24(3):612-618.
- [Google Scholar]
- Clinical features of migraine aura: results from a prospective diary-aided study. Cephalalgia. 2017;37(10):979-989.
- [Google Scholar]
- Variability of clinical features in attacks of migraine with aura. Cephalalgia. 2016;36(3):216-224.
- [Google Scholar]
- Clinical features of visual migraine aura: a systematic review. J Headache Pain. 2019;20(1):64-70.
- [Google Scholar]
- A nosographic analysis of the migraine aura in a general population. Brain. 1996;119:355-361. (Pt 2)
- [Google Scholar]
- The international classification of headache disorders, 3rd edition (ICHD-3). Cephalalgia. 2013;33:629-808.
- [Google Scholar]
- The effect of patent foramen ovale closure on visual aura without headache or typical aura with migraine headache. JACC Cardiovasc Interv. 2012;5(6):682-687.
- [Google Scholar]
- Persistent negative visual aura in migraine without headache: a case report. J Med Case Reports. 2014;8(1):61.
- [Google Scholar]
- Headache, cerebrovascular symptoms, and stroke: the Atherosclerosis Risk in Communities Study. Neurology. 2005;64(9):1573-1577.
- [Google Scholar]
- Migraine with visual aura is a risk factor for incident atrial fibrillation: a cohort study. Neurology. 2018;91(24):e2202-e2210.
- [Google Scholar]
- Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ. 2018;360:k96.
- [Google Scholar]
- Headache pattern in India—a headache clinic analysis of 1000 patients. Cephalalgia. 1997;17:316-317.
- [Google Scholar]
- The aura: a tertiary care study of 952 migraine patients. Cephalalgia. 2004;24(9):728-734.
- [Google Scholar]
- Observations on prodromes of classic migraine in a headache clinic population. Headache. 1986;26(5):216-219.
- [Google Scholar]
- Classic migraine—clinical findings in 164 patients. Eur Neurol. 1985;24(3):163-169.
- [Google Scholar]
- A prospective study of the headache phase in 32 migraine with aura patients. Cephalalgia. 2002;22(6):411-415.
- [Google Scholar]
- Characteristics of migraine visual aura in Southern Brazil and Northern USA. Cephalalgia. 2011;31(16):1652-1658.
- [Google Scholar]
- The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25(10):801-810.
- [Google Scholar]
- Cerebral and extracranial circulatory disturbances in migraine: pathophysiological implications. Cerebrovasc Brain Metab Rev. 1991;3(1):1-28.
- [Google Scholar]
- Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123(7):612-624.
- [Google Scholar]
- Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open. 2018;8(3):e020498.
- [Google Scholar]
- Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830-833. (6983)
- [Google Scholar]
- Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343-349.
- [Google Scholar]
- Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31(1):17-35.
- [Google Scholar]
- Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117:199-210. (Pt 1)
- [Google Scholar]
- Elementary visual hallucinations in migraine and epilepsy. J Neurol Neurosurg Psychiatry. 1994;57(11):1371-1374.
- [Google Scholar]
- Elementary visual hallucinations, blindness, and headache in idiopathic occipital epilepsy: differentiation from migraine. J Neurol Neurosurg Psychiatry. 1999;66(4):536-540.
- [Google Scholar]
- Improved description of the migraine aura by a diagnostic aura diary. Cephalalgia. 1994;14(2):107-117.
- [Google Scholar]






