Translate this page into:
Vascular Manifestations of Tuberculous Meningitis: MR Angiography and Venography Study
Suprava Naik, MD Department of Radiodiagnosis, All India Institute of Medical Sciences Bhubaneswar, Odisha 751019 India drsuprava.rd@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Purpose The purpose of this study is to evaluate magnetic resonance (MR) angiography (MRA) and venography (MRV) findings in tuberculous meningitis (TBM).
Methods Thirty consecutive patients of clinically diagnosed TBM were enrolled. Apart from T2-weighted imaging, T1-weighted imaging (T1WI), diffusion-weighted imaging, susceptibility-weighted imaging, fluid-attenuated inversion recovery, and postcontrast T1WI, time-of-flight (TOF) MRA and postcontrast MRV were done in all the patients. MRV was done after intravenous administration of gadolinium-based contrast agent followed by postcontrast T1WI. MRA and MRV findings were analyzed.
Results Mean age of the patients was 33.13 ± 14.93 years. Duration of symptom was 34.90 ± 33.82 (range: 10–150) days. Out of 30 patients, 11 were categorized as definite TBM and 19 probable TBM. Eighteen (60%) were grade I, 7 (23%) grade II, and 5 (16%) grade III TBM based on severity. MR abnormalities were in varying combinations of leptomeningeal enhancement in 24 (80%), pachymeningeal in 2, both in 3, tuberculomas in 13 (43.3%), ventriculitis in 1, hydrocephalus in 16 (53.3%), and infarcts in 10 (33.3%) patients out of which the tubercular zone infarct in 9 patients. TOF MRA showed arterial abnormality in 13 patients. Anterior cerebral artery and middle cerebral artery have commonly involved vessels. Dural sinus thrombosis was noted in two patients. Both were female. One patient had subacute thrombus in the posterior part of superior sagittal sinus, left transverse sinus, and proximal right transverse sinus. The second patient had a filling defect in the transverse sinus.
Conclusion In TBM, there is predominant arterial involvement causing infarcts which are usually seen in the tubercular zone. However, occasionally, there may be venous involvement causing cerebral venous sinus thrombosis.
Keywords
meningitis
MRV
cerebral venous sinus thrombosis
TBM
Introduction
Tuberculous meningitis (TBM) is the most severe form of tuberculosis (TB) and a major health problem in developing countries. Central nervous system (CNS) TB accounts for 1% of all cases of TB and is a major cause of morbidity and mortality.1 Clinical spectrum of intracranial TB includes basal meningitis, leptomeningitis, pachymeningitis, tuberculomas, tubercular abscess, ventriculitis, hydrocephalus, and infarcts.2 Cerebral venous sinus thrombosis (CVST) has been reported in other CNS infections. The presence of sinus thrombosis in CNS infection also increases the risk of death and disability in these patients.3 Arterial narrowing and infarcts are already established in TBM. There is a paucity of studies about the occurrence of CVST in TBM patients. In this article, we have analyzed arterial and venous sinus abnormality on magnetic resonance imaging (MRI) in TBM patients.
Materials and Methods
This is a cross-sectional observational study conducted in a tertiary care teaching institute after approval from the institute's ethics committee. Patients were recruited between January 2018 and April 2020 after obtaining written informed consent from the patient or their relatives.
Thirty consecutive patients with clinically diagnosed TBM were enrolled in this study. Detailed clinical evaluation, duration of illness, presence of seizure, focal neurological deficit, or altered sensorium was noted. Consciousness was assessed by Glasgow coma scale (GCS). Routine hematology and cerebrospinal fluid (CSF) examination were done in all the patients. Evidence of infective focus elsewhere in the body was evaluated that also added to the final diagnosis.
Diagnosis of TBM was made according to the following criteria4 5:
Definite TBM: Patients having clinical symptoms of meningitis for > 10 days and presence of acid-fast bacillus in CSF or culture or a positive cartridge-based nucleic acid amplification test or polymerase chain reaction in CSF.
Probable TBM: Patients having clinical symptoms of meningitis for > 10 days with at least two of the following supportive criteria:
-
More than 20 cells in CSF with lymphocytic predominance, raised protein
-
MRI findings of TBM (basal exudates, meningeal enhancement, hydrocephalous, vasculitic infarcts, tuberculoma in various combinations)
-
Presence of TB elsewhere in the body.
Based on the severity of meningitis, TBM cases were categorized as stage I (meningitis only), stage II (meningitis with focal neurological deficit or GCS score of 11–14), and stage III (meningitis with GCS score < 11).6
Imaging: MRI of the brain was done in a 1.5-T MRI scanner (Siemens Magnetom Aera) using 20 channel head coil. Apart from routine T2-weighted imaging, T1-weighted imaging (T1WI), diffusion-weighted imaging, susceptibility-weighted imaging, fluid-attenuated inversion recovery, and postcontrast T1WI, three-dimensional (3D) time-of-flight MR angiography (TOF MRA) and contrast-enhanced MR venography (MRV) was done in all the patients. 3D TOF MRA was done using repetition time (TR) 24, echo time (TE) 7, and flip angle of 25 degrees. Postcontrast MRV was done after intravenous administration of 0.1 mg/kg dose gadolinium-based contrast agent (Magnilek) by care bolus technique with TR 3.1, TE 1.1 milliseconds. Postcontrast T1WI was also acquired after MRV. Postprocessing was done on the multipurpose workstation of Siemens.
MRI findings were analyzed for the presence or absence of leptomeningeal enhancement, pachymeningeal enhancement, basal exudates, tuberculomas, abscess, ventriculitis, hydrocephalus, and vasculitic infarcts. The location of the cerebral infarcts was documented. The location and characteristics of tuberculoma were noted. MRA and MRV abnormalities were also analyzed by two radiologists with more than 11 years of experience. Area of irregularity, narrowing, and the arteries involved were documented for major intracranial arteries. Arterial narrowing of more than 50% compared with the proximal normal arterial segment was considered as significant narrowing. The presence and location of infarcts were noted. Infarcts were categorized in the tubercular zone (caudate, lentiform nuclei, anterior limb and genu of internal capsule, and anterior thalamus) and ischemic zone (putamen, posterior limb of internal capsule, and posterolateral thalamus). Opacification of major dural sinuses on postcontrast venography and the presence of flow void in conventional sequences were considered normal. Hypoplastic right or left transverse sinus was also considered as a normal variant. Filling defects in the dural sinuses or deep cerebral veins were considered abnormal. The focal filling defect could be due to arachnoid granulations within the dural sinuses which are commonly seen in superior sagittal sinus and transverse sinus. If the focal filling defect within the sinus was linear and irregular, it was considered abnormal. MRV findings were correlated with signal in T1WI and T2WI in the corresponding areas. Statistical analysis was performed using SPSS version 15 (Chicago, Illinois, United States).
Results
A total of 30 patients with clinical features and CSF findings of confirmed or probable TBM were included in the study. The mean age of the patients was 33.13 ± 14.93 (range: 18–65 years); 56.7% were female. Duration of symptom was 34.90 ± 33.82 (range: 10–150) days. Details of demographic, clinical, and radiological findings in 30 TBM patients are described in Table 1. Out of the 30 TBM patients, 11 (36.7%) were categorized as definite TBM and 19 (63.3%) probable TBM. According to grade of TBM, 18 (60%) were grade I, 7 (23%) grade II, and 5 (16%) grade III TBM.
|
Variables |
N = 30 (%) |
|---|---|
|
Mean age (in years) |
33.13 ± 14.93 (range: 18–65) |
|
Gender (female/male) |
17 (56.7)/13 (43.3) |
|
Clinical symptoms |
|
|
Duration of symptom (in days) |
34.90 ± 33.82 (10–150) |
|
Fever |
30 (100%) |
|
Headache |
28 (93.3%) |
|
Vomiting |
17(56.7%) |
|
Seizure |
6 (20%) |
|
Altered sensorium |
19 (63.3%) |
|
Weakness |
13 (43.3%) |
|
Signs |
|
|
Neck stiffness |
29 (%) |
|
Kernig sign |
9 (30%) |
|
Papilledema |
8 (27%) |
|
GCS |
13.47 ± 2.22 |
|
Grade of TBM |
|
|
Grade 1 |
18/30 (60%) |
|
Grade 2 |
7 (23%) |
|
Grade 3 |
5 (16%) |
|
CSF findings |
|
|
Total cells (/mm3) |
249.72 ± 331.59 |
|
Lymphocytic predominance |
25/30 |
|
Protein (mg/dL) |
241.71 ± 162.85 |
|
Sugar (mg/dL) |
45.22 ± 25.86 |
|
ADA (IU/L) |
20.89 ± 23.13 |
|
CBNAAT/PCR |
9 |
|
AFB |
2 |
|
MRI findings |
|
|
Abnormal |
29 (%) |
|
Meningeal enhancement |
29 |
|
Pachy |
2 |
|
Lepto |
24 |
|
Both |
3 |
|
None |
1 |
|
Basal exudates |
21 (70%) |
|
Hydrocephalus |
16 (53.3%) |
|
Ventriculitis |
1 (3.3%) |
|
Tuberculoma |
13 (43.3%) |
|
Vasculitic infarcts |
10 (33.3%) |
|
Tubercular zone infarcts |
9 (30%) |
|
Ischemic zone infarcts |
1 (3.3%) |
|
MRA abnormalities |
13 (43.3%) |
|
MRV abnormalities |
2 (6.7%) |
|
Category TBM |
|
|
Confirmed TBM (CSF AFB/CBNAAT/PCR positive) |
11 (36.7%) |
|
Probable TBM |
19 (63.3%) |
|
Evidence of extra-CNS TB |
6 (20%) |
|
Clinical outcome at discharge |
|
|
Improved |
23 (76.7%) |
|
Death |
2 (6.7%) |
|
Bed bound |
5 (16.7%) |
|
Better outcome |
23 (76.7%) |
|
Poor outcome |
7 (23.3%) |
Abbreviations: ADA, adenosine deaminase; AFB, acid-fast bacillus; CBNAAT, cartridge-based nucleic acid amplification test; CNS, central nervous system; GCS, Glasgow coma scale; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; MRV, magnetic resonance venography; PCR, polymerase chain reaction; TB, tuberculosis; TBM, tuberculous meningitis.
Twenty-nine TBM (96.7%) patients have an abnormality on MRI. MR abnormalities were in varying combinations of leptomeningeal enhancement in 24 (80%), pachymeningeal enhancement in 2 (6.7%), both lepto- and pachymeningeal enhancement in 3 (10%), tuberculomas in 13 (43.3%), ventriculitis in 1 (3.3%), hydrocephalus in 16 (53.3%), and infarcts in 10 (33.3%), details given in Table 1. Cerebral infarcts were seen in 10 (33.3%) patients, out of which the tubercular zone is involved in 9 patients. The representative image is given in Fig. 1.
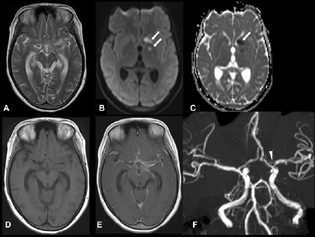
-
Fig. 1 Axial T2-weighted imaging (A) shows hyperintensity in the left perisylvian region and prominent temporal horn. Axial diffusion-weighted imaging (B) and apparent diffusion coefficient (C) show acute infarcts in the head of the left caudate and globus pallidum (arrows). Axial T1-weighted imaging (T1WI) (D) and postcontrast T1WI (E) show enhancement in the basal cisterns and bilateral Sylvian cistern. Time-of-flight magnetic resonance angiography (F) shows focal significant narrowing of the proximal left middle cerebral artery (arrowhead).
Fig. 1 Axial T2-weighted imaging (A) shows hyperintensity in the left perisylvian region and prominent temporal horn. Axial diffusion-weighted imaging (B) and apparent diffusion coefficient (C) show acute infarcts in the head of the left caudate and globus pallidum (arrows). Axial T1-weighted imaging (T1WI) (D) and postcontrast T1WI (E) show enhancement in the basal cisterns and bilateral Sylvian cistern. Time-of-flight magnetic resonance angiography (F) shows focal significant narrowing of the proximal left middle cerebral artery (arrowhead).
Arterial abnormality on TOF MRA was noted in 13 (43.3%) patients. The significant arterial narrowing was noted in four patients, whereas mild irregularity of vessels was noted in nine patients. Anterior cerebral artery (ACA) was involved in five patients (bilateral in three and unilateral in two), middle cerebral artery (MCA) in seven patients (bilateral in two and unilateral in five), both MCA and ACA in two patients (left side), PCA in two (unilateral, one on right and one left), and supraclinoid internal carotid artery in two patients (unilateral on the right side).
Comparison of clinical and MRI parameters between TBM patients having normal and abnormal MRA findings is given in Table 2. GCS at admission was significantly lower in patients having abnormality in MRA compared with those without abnormality in MRA (p = 0.05). This was attributed to the presence of vasculitic infarcts in patients with abnormal MRA findings. Basal exudates and hydrocephalous were also significantly greater in patients with abnormal MRA findings (p = 0.02 and p = 0.03, respectively). The outcome is better in patients with normal MRA findings.
|
Abnormal MRA (n = 13) |
Normal MRA (n = 17) |
p-Value |
|
|---|---|---|---|
|
Age (years) |
32.77 ± 12.84 |
33.41 ± 16.73 |
0.91 |
|
Gender |
Male 5, female 8 |
Male 8, female 9 |
|
|
Duration of illness (days) |
29.77 ± 27.98 |
38.82 ± 38.05 |
0.46 |
|
Seizure |
2 |
4 |
0.59 |
|
GCS at admission |
12 ± 2 |
13.44 ± 1.75 |
0.053 |
|
Weakness |
9 (69.2%) |
4 (23.5%) |
0.01 |
|
Altered sensorium |
8 |
11 (64.7%) |
0.86 |
|
CSF |
|||
|
Cells |
127.89 ± 108.277 |
318.25 ± 394.661 |
0.051 |
|
Proteins (/mm3) |
187.7 ± 89.10 |
280.286 ± 193.85 |
0.253 |
|
Definite TBM (mg/dL) |
7 (53.8%) |
4 (30.8%) |
0.79 |
|
Meningeal enhancement |
13 (100%) |
16(94.1%) |
0.382 |
|
Basal exudates |
12 (92.3%) |
9 (52.9%) |
0.02 |
|
Hydrocephalus |
10 (76.9%) |
6 (35.3%) |
0.03 |
|
Ventriculitis |
0 |
1 (5.9%) |
0.38 |
|
Tuberculoma |
6 (46.2%) |
7 (41.2%) |
0.79 |
|
Vasculitic infarct |
7 (53.8%) |
3 (17.6%) |
0.07 |
|
Outcome at discharge |
9 (69.2%) |
15 (88.2%) |
0.17 |
|
Meningitis grade |
|||
|
Grade 1 |
5 (38.5%) |
12 (70.6%) |
0.16 |
|
Grade 2 |
5 (38.5%) |
2(11.8%) |
|
|
Grade 3 |
3 (23.1%) |
3 (17.6%) |
|
Abbreviations: CSF, cerebrospinal fluid; GCS, Glasgow coma scale; MRA, magnetic resonance angiography; TBM, tuberculous meningitis.
Abnormal MRV was noted in two (6.7%) of patients. Both were female. One patient had subacute thrombus in the posterior part of the superior sagittal sinus, left transverse sinus, and proximal right transverse sinus (Fig. 2). The second patient had a partial filling defect in the nondominant transverse sinus. Details of the patients are given in Table 3. Both of our TBM patients having sinus thrombosis improved after treatment. Two patients had hypoplastic left transverse sinus.
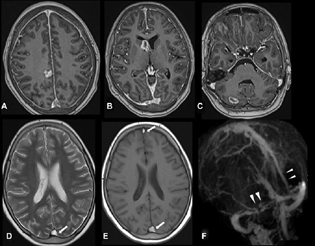
-
Fig. 2 Axial postcontrast three-dimensional T1-weighted imaging (T1WI) sequence (A–C) shows multiple nodular and ring-enhancing lesions in the right frontal lobe, head of right caudate, and right cerebellum. Diffuse pachymeningeal enhancement was also noted. Axial T2-weighted imaging (D) and T1WI (E) show hyperintense thrombus in the posterior and anterior parts of the superior sagittal sinus (SSS) (arrows). Postcontrast magnetic resonance venography (F) shows filling defects in the posterior and anterior parts of SSS, right transverse sinus, and proximal left transverse sinus (arrowheads).
Fig. 2 Axial postcontrast three-dimensional T1-weighted imaging (T1WI) sequence (A–C) shows multiple nodular and ring-enhancing lesions in the right frontal lobe, head of right caudate, and right cerebellum. Diffuse pachymeningeal enhancement was also noted. Axial T2-weighted imaging (D) and T1WI (E) show hyperintense thrombus in the posterior and anterior parts of the superior sagittal sinus (SSS) (arrows). Postcontrast magnetic resonance venography (F) shows filling defects in the posterior and anterior parts of SSS, right transverse sinus, and proximal left transverse sinus (arrowheads).
|
Parameters |
Patient 1 |
Patient 2 |
|---|---|---|
|
Age, y/sex |
23 y/F |
27 y/F |
|
Definite TBM |
Definite TBM |
|
|
Grade of TBM |
Grade 1 |
Grade 1 |
|
Clinical presentation |
Low-grade fever for 3 mo, holocranial headache for 3 mo, loss of vision in both eyes for 20 d Bilateral papilledema present, DTR normal, bilateral plantar flexor, neck stiffness and Kernig sign was positive Hb 10.2 g%, TLC 9,200/mm3, and ESR was 34 mm at the end of 1 h |
Low-grade fever, severe headache, and intermittent vomiting for 4 mo; blurring of vision for 2 mo Papilledema on right side, left optic atrophy Neck stiffness and Kernig sign was positive Hb 12.2 g%, TLC 11,870/mm3, and ESR was 40 mm at the end of 1 h |
|
CSF |
||
|
Protein |
96 |
86 |
|
Sugar (mg/dL) |
20 |
53 |
|
Cells/mm3 |
36 (lymphocytic predominance) |
94 (lymphocytic predominance) |
|
CBNAAT/PCR |
Positive |
Positive |
|
MRI findings |
||
|
Meningeal enhancement |
Diffuse pachymeningeal enhancement (predominantly frontoparietal) |
Present (leptomeningeal enhancement) |
|
Basal exudate |
Absent |
Present (perimesencephalic, prepontine cistern, and prechiasmal region) |
|
Tuberculoma |
Present (right caudate, frontal lobe, and right cerebellum) |
Absent |
|
Hydrocephalous |
Absent |
Present |
|
Infarcts |
Absent |
Absent |
|
MRA abnormality |
Absent |
Mild irregularity and narrowing of right cavernous, supraclinoid, and prox MCA |
|
MRV abnormality |
Present (filling defect in posterior aspect of superior sagittal sinus, left transverse sinus, and small part of right transverse sinus. Corresponding region shows loss of flow void and appears hyperintense on both T1W and T2W s/o subacute thrombus (Fig. 2) |
Partial linear filling defect in right transverse and sigmoid sinus suggestive of thrombosis |
|
Treatment |
Antituberculous treatment (ATT) 3 d pulse methylprednisolone IV followed by oral steroid LMWH for 10 d |
ATT 3 d pulse methylprednisolone IV followed by oral steroid LMWH for 10 d |
|
Outcome |
Improved at discharge |
Improved clinically at discharge. Vision improved on right, but did not improve on left |
Abbreviations: ATT, antituberculous treatment; CBNAAT, cartridge-based nucleic acid amplification test; CSF, cerebrospinal fluid; DTR, deep tendon reflex; ESR, erythrocyte sedimentation rate; IV, intravenous; LMWH, low-molecular-weight heparin; MCA, middle cerebral artery; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; MRV, magnetic resonance venography; PCR, polymerase chain reaction; TBM, tuberculous meningitis; TLC, total leukocyte count.
Discussion
TBM is the most severe form of extrapulmonary TB. Cerebrovascular complications in TBM have been reported as a poor prognostic predictor. Vascular complications associated with TBM are usually secondary to arteritis causing arterial vasculitic infarcts. We found cerebral infarcts in 10 out of 30 (33.3%) cases. Arterial narrowing and abnormalities were found in 13 (43.3%) patients in our study. Infarcts were noted in 10 (33.3%) out of which 9 were in the tubercular zone. Cerebrovascular infarcts are complications of TBM which occurs in 15 to 57% of patients.7 In a study by Kalita et al, they found cerebral infarct in 50% of cases of TBM.8 In another retrospective study by Soni et al, they found cerebral infarcts in 57.7% of TBM patients.9 Cerebral infarcts in TBM are common and predominantly involve the perforator and cortical territory. In our study, most of the infarcts were in the tubercular zone which is like various previous studies. Arterial stroke has been reported in 15 to 67% of TBM patients and is associated with poor outcomes.7 8 9 10 11 12
Venous pathology following intracranial TB is very rare. We found CVST in 6.7% patients of with TBM. Infection accounts for less than 10% of all causes of CVST, although TBM is a rare cause. There are few case reports of CVST in TBM.13 14 15 In a large study by Bansod et al, abnormal MRV finding was noted in 12 (11.2%) out of 107 TBM patients.16 In another study by Kalita et al, there was no evidence of venous thrombosis, although many variations in sinuses such as hypoplastic transverse sinus were seen.8 Guenifi et al found 3 patients of CVST out of 61 patients of TBM.17
Infectious causes of CVST are usually secondary to pyogenic meningitis, most common being sigmoid sinus and cavernous sinus thrombosis secondary to mastoiditis and sinusitis, respectively.3 Its occurrence in TBM is rare. There is also overlap in the clinical picture of TBM and CVST. So, the clinical presentation of CVST can be confused and falsely attributed to TBM. Contrast-enhanced MRI and MRV are the diagnostic modality of choice for TBM and CVST. Postcontrast T1WI shows meningeal enhancement, basal exudates which are features of tubercular meningitis. MRI can also show complications such as hydrocephalus and the mass effect of tuberculoma or tubercular abscess over the adjacent brain parenchyma. In CVST, there is loss of flow void in the dural sinuses in T1WI and T2WI and filling defects are seen in MRV.18
The pathophysiologic mechanism of CVST in the case of TBM may be due to endothelial injury, alteration in normal blood flow, and in blood coagulability. The hypercoagulable state occurs in patients with TB because they show increased platelet aggregability. Patients with underlying hypercoagulable states precipitate the occurrence of a thrombotic event.14 19 TBM or tubercular exudate may cause endothelial injury by inflammatory infiltrates in their wall as it affects arteries. Alteration in prothrombotic status (protein C, protein S, antiphospholipid antibody syndrome) and increase in proinflammatory cytokines such as tumor necrosis factor-α- and interleukin-6-secreting cells in peripheral blood mononuclear cells has been reported in patients infected with Mycobacterium tuberculosis.20 In a histopathological study by Chatterjee et al, in 9 out of 51 cases of TBM, there was thrombophlebitis affecting medium-sized meningeal cortical veins.21
Venous abnormality in TBM is rare, although many cases and series have been reported. Timely management of TBM associated with CVST is important for a better prognosis. It is not always associated with poor outcomes if early treatment is provided. Antitubercular drugs are the mainstay of treatment in TBM with associated CVST. The role of anticoagulation in infective CVST is less defined. All the patients were treated with a standard antitubercular regimen and corticosteroid. Both the patients with CVST in our study were managed with an injection of low-molecular-weight heparin followed by oral anticoagulation tapered over 3 months. Both the patients were stable at the time of discharge and improved at 6 months follow-up.
The strength of our study is that it is a prospective study. We have done postcontrast MRV in all cases. However, this study has a few limitations. The sample size is small. Only adult patients were included. The number of confirmed TBM was comparatively less. Predisposing factors for CVST were not done in all patients. A larger study is required to know any association of tubercular meningitis with CVST.
Conclusion
CVST in tubercular meningitis is rare and the clinical pictures may be overlapping. Early diagnosis and management are required for better outcome of the patient. Contrast-enhanced MRV with postcontrast T1WI is required for diagnosis of sinus thrombosis and meningitis. In TBM, there is predominant arterial involvement causing infarcts. However, occasionally, there may be venous involvement causing CVST.
Conflict of Interest
None declared.
Funding Institutional intramural grant was received.
References
- CNS tuberculosis: a longitudinal analysis of epidemiological and clinical features. Int J Tuberc Lung Dis. 2006;10(1):99-103.
- [Google Scholar]
- Central nervous system manifestations of tuberculosis: a review article. J Mycobac Dis. 2014;4:146.
- [Google Scholar]
- Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35(3):664-670.
- [Google Scholar]
- Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741-1751.
- [Google Scholar]
- Does adjunctive corticosteroid and aspirin therapy improve the outcome of tuberculous meningitis? Neurol India. 2018;66(6):1672-1677.
- [Google Scholar]
- Predictors of stroke and its significance in the outcome of tuberculous meningitis. J Stroke Cerebrovasc Dis. 2009;18(4):251-258.
- [Google Scholar]
- Evaluation of cerebral arterial and venous system in tuberculous meningitis. J Neuroradiol. 2018;45(2):130-135.
- [Google Scholar]
- Cerebrovascular complications in tuberculous meningitis-a magnetic resonance imaging study in 90 patients from a tertiary care hospital. Neuroradiol J. 2020;33(1):3-16.
- [Google Scholar]
- Magnetic resonance angiography manifestations and prognostic significance in HIV-negative tuberculosis meningitis. Int J Tuberc Lung Dis. 2015;19(12):1448-1454.
- [Google Scholar]
- Frequency and impact of cerebral infarctions in patients with tuberculous meningitis. Stroke. 2018;49(10):2288-2293.
- [Google Scholar]
- A rare presentation of cerebral venous sinus thrombosis associated with tubercular meningitis. BMJ Case Rep. 2013;2013:bcr2013009892.
- [Google Scholar]
- Tuberculosis: an uncommon cause of cerebral venous thrombosis? Arq Neuropsiquiatr. 2005;63:852-854. (3B):
- [Google Scholar]
- An unusual association: tuberculous meningitis causing cerebral venous sinus thrombosis. J Case Report. 2015;20(2):343-346. ;5
- [Google Scholar]
- Magnetic resonance venographic findings in patients with tuberculous meningitis: predictors and outcome. Magn Reson Imaging. 2018;54:8-14.
- [Google Scholar]
- Cerebral venous thrombosis during tuberculous meningoencephalitis [in French] J Mal Vasc. 2016;41(3):210-214.
- [Google Scholar]
- Acute-phase response and the hypercoagulable state in pulmonary tuberculosis. Br J Haematol. 1996;93(4):943-949.
- [Google Scholar]
- Increase in tumor necrosis factor alpha- and interleukin-6-secreting cells in peripheral blood mononuclear cells from subjects infected with Mycobacterium tuberculosis. Infect Immun. 1991;59(9):3021-3025.
- [Google Scholar]
- Vascular complications of tuberculous meningitis: An autopsy study. Neurol India. 2015;63(6):926-932.
- [Google Scholar]







