Translate this page into:
Use of cannabidiol in the treatment of drug-refractory epilepsy in children and young adults: A systematic review
*Corresponding author: Samuel Fernando Vargas Chico, Department of Medicine, Rafael Nuñez University Corporation, Cartagena, Colombia. svargasc044@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vargas Chico SF, Melendez Diaz DA, ContrerasPuentes N. Use of cannabidiol in the treatment of drug-refractory epilepsy in children and young adults: A systematic review. J Neurosci Rural Pract. 2024;15:203-10. doi: 10.25259/JNRP_618_2023
Abstract
Objectives:
Epilepsy poses a significant challenge in pediatric and adolescent populations, impacting not only seizures but also psychological and cognitive comorbidities, leading to higher mortality rates than the general population. Drug-refractory epilepsy, resistant to conventional treatments, affects a range of 7–20% of pediatric patients. The search for alternative therapies has led to exploring the therapeutic potential of Cannabis sativa L. compounds, particularly cannabidiol (CBD). Examine the use of CBD for treating drug-refractory epilepsy in children and young adults, summarizing existing evidence on its efficacy.
Materials and Methods:
A systematic review, following Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines, assessed studies from 2018 to 2023, focusing on CBD’s efficacy and safety for treatment-resistant epilepsy in pediatric and juvenile populations. The search spanned seven databases, and the studies underwent rigorous screening and data extraction.
Results:
Out of 6351 identified articles, eight were selected for review. The included studies reported positive outcomes, with CBD leading to a reduction in seizure frequency ranging from 50% to complete seizure freedom. Adverse effects were mostly mild and reversible, including drowsiness, diarrhea, and loss of appetite.
Conclusion:
The CBD emerges as a promising tool for refractory epilepsy in pediatric patients, showing efficacy in reducing seizure frequency and improving overall quality of life. Despite mild and reversible adverse effects, CBD’s benefits outweigh the risks. However, more research on long-term effects is needed to fully understand its implications.
Keywords
Refractory epilepsy
Children
Young
Cannabidiol
Cannabis
INTRODUCTION
Epilepsy is among the neurological pathologies that most frequently affect pediatric and adolescent patients, accompanied by various psychological, behavioral, and cognitive comorbidities that profoundly impact the quality of life of the patients;[1] Among these, intellectual disability, attention deficit hyperactivity disorder, autism, anxiety, depression, and sleep disorders stand out.[2] The condition of epilepsy is characterized by the presence of irregular neuronal discharges or abnormal overexcitation of neurons with synchronization; it is classified into three subtypes: Acquired, idiopathic, and epilepsy of genetic origin with approximately 1000 linked genes. The essence of the pathology lies in the influence of inhibition through g-aminobutyric acid (GABA) as well as glutamate-mediated stimulation.[3] Moreover, mortality rates in pediatric and young patients with epilepsy are 5–10 times higher than in the general population. This substantial increase in risk is attributed to neurometabolic conditions, systemic complications, and neuro disability.[4]
The international league against epilepsy defines treatment-resistant epilepsy as the lack of a successful response to the administration of two antiepileptic drug regimens.[5] It is estimated that 30% of pediatric patients are resistant to conventional drug treatments.[6] Considering that treatment-resistant epilepsy may impact pediatric populations at a rate ranging from 7% to 20%.[7] Conversely, the likelihood of refractoriness increases in the presence of epileptic syndromes such as Lennox–Gastaut, Dravet syndrome, and tuberous sclerosis, as well as other underlying etiologies. These conditions might exhibit an unfavorable clinical outcome for patients due to their severity, early onset, and slow progression.[8,9]
The need for novel therapies and the lack of response to antiepileptic drugs have positioned scientific research in a critical role in the development of new approaches that enhance the quality of life for patients, such as the therapeutic use of Cannabis sativa L. and its metabolites. To date, 566 chemical compounds have been isolated from cannabis, including 125 cannabinoids and 198 non-cannabinoids. Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are the cannabinoids with the highest concentration.[10,11] THC is a cannabinoid with psychoactive activity, whereas CBD lacks such activity. Nevertheless, it exhibits various therapeutic properties; experimental studies have identified anticonvulsant, analgesic, anxiolytic, antiemetic, immunomodulatory, anti-inflammatory, neuroprotective, and antitumorigenic effects.[12]
Knowledge of the use of cannabis products for the treatment of epilepsy dates back to ancient times; however, it was not until the 1990s that CBD began to be employed as a treatment option for this condition. This was deemed advantageous due to its anticonvulsant activity without inducing psychoactive effects. The versatile applications and safety profile of CBD led to its approval in the United States in 2018 under the trade name Epidiolex.[13] Furthermore, it is worth noting that this type of product has expanded its coverage and usage due to its efficacy in reducing the frequency of epileptic seizures for different types of epilepsy, such as tonic, tonic-clonic, epileptic encephalopathy, focal seizures, and generalized seizures. However, it is important to mention that, while it has benefits, it can also generate adverse effects, such as drowsiness, decreased appetite, elevated liver transaminases, fatigue, diarrhea, changes in behavior, and sleep disorders.[14]
This systematic review provides updated information on the efficacy and safety of the therapeutic use of CBD for treatment-resistant epilepsy in the pediatric and juvenile populations.
MATERIALS AND METHODS
Type of study and search strategies
A systematic review was carried out following the statement’s guidelines for preferred reporting items for systematic reviews and meta-analyses.[15] The search was carried out through seven databases: PubMed (Medline), EBSCO, ScienceDirect, Scopus, Taylor and Francis, Web of Sciences, and Wiley Online Library. The studies were restricted to studies dated between 2018 and 2023, published in the English language, type of publication, and full-text articles only. The detailed search syntax is included in the Supplementary Material – S1 Appendix.
Study selection
Three investigators (SV, NC, and DM) independently screened the titles and abstracts. The articles in the full text were retrieved for relevant citations and reviewed for inclusion in the final analysis. The principal research authors (NC) have resolved some disagreements.
Data collection process and data extraction
Two investigators independently extracted data using a standard extraction template based on a table designed in Excel format, with discrepancies resolved by two additional authors (SV and DM). The information collected included the aim of the studies, type of study, dosing, and adverse events reported. Participant information included the number of participants, age, sex, and previously administered antiepileptic drugs. Information on the intervention included details of efficacy in the administration of CBD products reported in terms of the percent decrease in seizure crises. In addition, the principal adverse events in each study were collected.
Assessment of the risk of bias
The risk of bias was assessed using the Joana Briggs Institute’s critical appraisal tools. The questionnaires were applied to cohort studies, quasi-experimental, and analytical cross-sectional studies to evaluate the quality of the selected studies.
RESULTS
A total of 6351 articles were identified from the databases of EBSCO, PubMed, ScienceDirect, Scopus, Taylor and Francis, Web of Sciences, and Wiley Online. Of these, 1686 were duplicates and were therefore eliminated. A total of 4665 articles were evaluated according to title and abstract and discarded. Of the 21 articles obtained, exclusion criteria were applied to obtain eight articles at the end [Table 1 and Figure 1].[16-23]
| Authors and year of publication | Study type | Population | Age (mean±SD) | Gender | Doses | Efficacy results | Adverse events |
|---|---|---|---|---|---|---|---|
| Pietrafusa et al., 2019[16] |
Open-label, prospective, and single-center study | 29 patients | Median: 9.3 years Range: 1.9–16.3 years SD: ± 4.7 |
12 males (41.4%) 17 females (48.6%) |
CBD extract+triglyceride oil. CBD dosage: 5 and 25 mg/kg/day. Mean dosage: 13.62±6 mg/kg/day. | CBD demonstrated a 50% reduction in seizure frequency, with one patient becoming seizure-free, and improvements in sleep, behavior, and cognitive functions. | Drowsiness, decreased appetite, and diarrhea, with no severe adverse effects reported in any patient |
| Marchese et al., 2022[17] |
Retrospective open-label study | 37 patients | Median: 16.1 Year Range: 2–54 years SD: ±12.3 years |
20 males (54%) 17 females (46%) |
Three daily sublingual doses of CBD oil (24%). Initial dose: 5–10 mg/Kg/day. Maximum dose: 50 mg/Kg/day) |
Seizure-free patients (19%). Seizure improvement >50% (73%). Seizure improvement <50% (5%). |
They were mild and temporary (25%). Drowsiness (77%) and loss of appetite (23%). |
| Chen et al., 2018[18] | Prospective open cohort study. | 40 patients | Median 8.4 years Range: 1.6–16.6 years | 22 boys (55%) 18 girls (45%) |
CBD oral solution (>98% purity; 100 mg/mL) Initial dose (5 mg/Kg/day) - two doses. Target dose: 25 mg/Kg/day). |
Thirty percent of caregivers reported improved general health, improved alertness (25-39%), improved language (8–11%), and improved sleep (7%). | Mild-to-moderate. benign viral illness (60%), increased seizures (40%), drowsiness (37.5%), diarrhea (22.5%), anorexia (17.5%), vomiting (17.5%), and rash (10%). |
| Caraballo et al., 2020[19] |
Prospective cohort study | 50 patients | Median: 10,5 years Range: 2–16 years | does not specify | Cannabis oil (95% CBD) (CBD/. THC ratio 27:1). Initial CBD dose: 2 mg/Kg/day, twice daily in children ≤45 kg, and 5 mg/Kg/day for >45 kg. Maximum dose: 25 mg/Kg/day. |
80% showed ↓ the frequency of seizures (959–381 seizures). And specially in encephalopathy epilepsy specifically the Dravet syndrome, lennox-gastaut syndrome Dalla Bernardina syndrome, and syndrome Doose |
Mild-to-moderate. Drowsiness (32%), loss of appetite, diarrhea, irritability or behavioral problems, weight loss, nausea, vomiting, mood swings, insomnia, blurred vision, dry mouth, and fever. |
| Hausman–Kedem et al., 2018[20] | Observational longitudinal study | 57 patients | Median 9.6 years SD: ±4.9 Range: 1–20 years |
does not specify | Oral CBD doses Median CBD dose: 11 mg/kg/day. Total daily doses: 77 and 800 mg (mean 305 mg, SD 136 mg, SD 136 mg). |
Reduction in monthly seizure frequency of 50% (56% of patients). Reduction rate of 75% (35% of patients). |
46% of patients presented with somnolence, aggressiveness, loss of appetite, vomiting, irritability, psychosis, depression, memory loss, movement disorder, reduced water intake, diarrhea, and exacerbation of seizures. |
| Herlopian et al., 2020[21] |
Open trial | 9 patients. | Median: 9 years Range: 2–16 years |
4 males (44.4%) y 5 females (55.5%) | GWP42003-P (CBD a 100 mg/mL). Initial dose: 5 mg/kg/day in two doses. Dose increase: 5 mg/Kg/day (weekly). Maximum dose: 50 mg/Kg/day. |
Success rate: ↓ epileptic spasms: 33% (week 2)–56% (week 12). EEG changes, seizure reduction/freedom, and subjective cognitive changes. |
Drowsiness, diarrhea, ataxia, loss of appetite, agitation, spasms, irritability, and elevated liver enzymes. |
| Miller et al., 2020[22] | Randomized clinical trial | 198 patients. | Median: 9.3 years Range: De 2 y 18 years. SD: 4.4 |
104 females (52.5%) y 94 males (47.5%). | 66 patients: 10 mg/kg/day dose of CBD (CBD10), and 67 patients, 20 mg/kg/day dose of CBD (CBD20). | Reduction of seizure frequencies (CBD10: 56.4%) and (CBD20: 47.3%). six patients got rid of seizures together with improvement of general condition. | Decreased appetite, diarrhea, drowsiness, pyrexia, and fatigue. |
| Patel et al., 2021[23] | Long-term OLE trial | 366 patients. | Range: 2 a 55 years Median: 16 years SD: 9.5 |
198 males (54%) 168 females (46%) | Purified CBD (100 mg/mL): 2.5–20 mg/Kg/day in two doses divided into 2 weeks. | Median seizure frequency (48%) in weeks 1–12; 5% of patient’s seizure-free in the last 12 weeks of treatment. Half of the individuals reduced seizure frequency in the first months (48–65%). | Convulsions, diarrhea, pyrexia, drowsiness, vomiting, respiratory tract infection, decreased appetite, cough, weight loss, nasopharyngitis, pneumonia, urinary tract infection, ear infection, sinusitis, nasal congestion, influenza, constipation, status epilepticus, insomnia, and fatigue. |
THC: Tetrahydrocannabinol CBD: Cannabidiol, SD: Standard deviation, OLE: Open-label extension
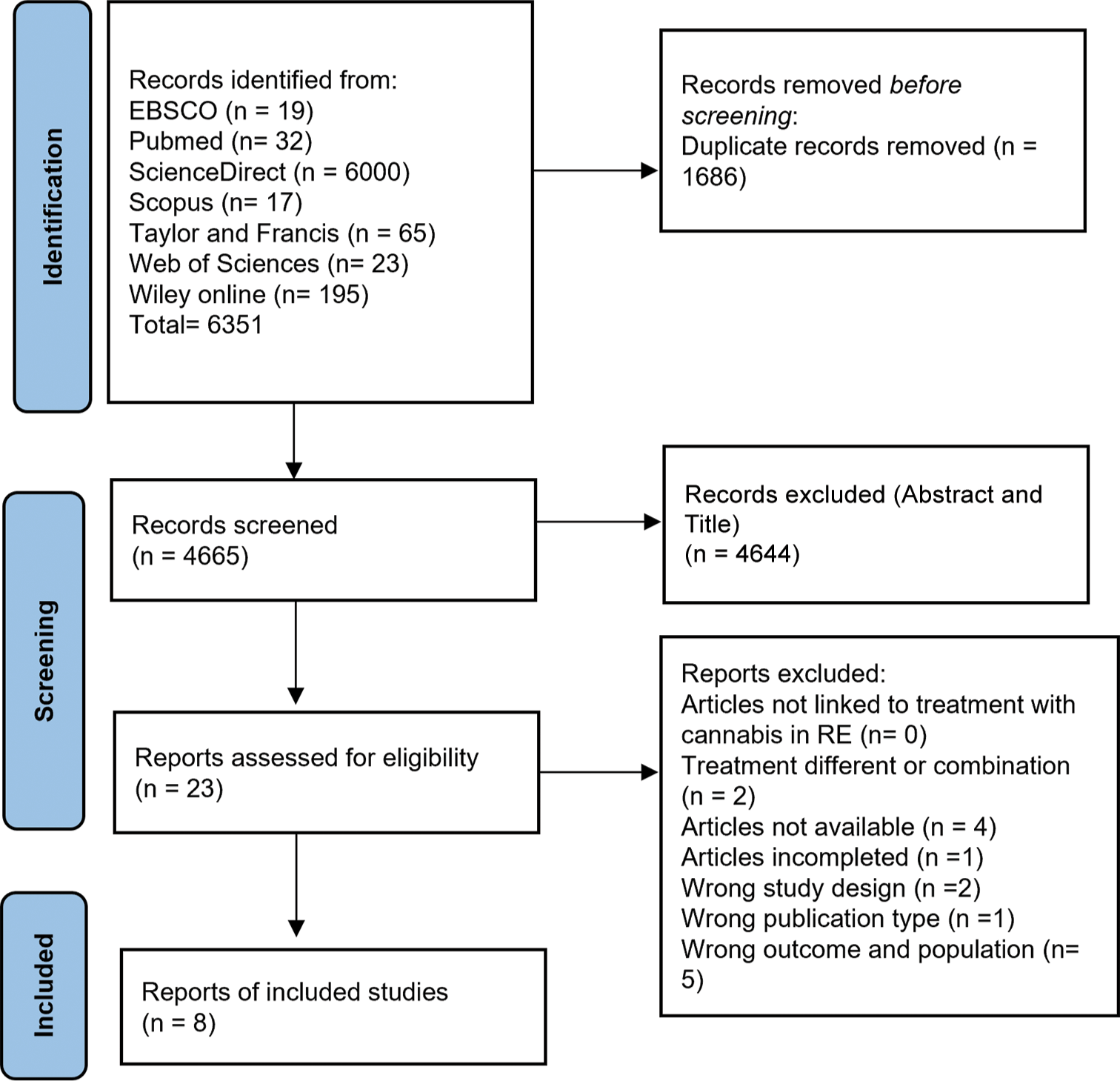
- Preferred reporting items for systematic reviews and meta-analyses flowchart on the strategy for selecting eligible studies about refractory epilepsy (RE) in pediatric and young people treated with cannabidiol.
Stoppage and frequency of seizures
Seven out of the eight studies had expected results, but the study by Chen et al. reported that none of the patients were free of seizures.[18] In all articles addressing the efficacy of CBD treatment in the context of refractory epilepsy, a reduction in seizures has been observed. From these studies, the overall response rates obtained after administration of CBD treatment during different follow-up periods, including 2 weeks, 1 month, and 12 months, were observed to be 67%, 78%, and 78%, respectively.
In the study by Pietrafusa, 37.9% presented an improvement over 50%.[16] In the studies of Caraballo et al. 80% responded positively, showing a notable decrease in seizure frequency.[19] Overall, 77.6% of patients had a 25% reduction, while 73.5% had at least a 50% reduction, and 49% achieved at least a 75% reduction in seizure frequency.
The study by Chen et al., on the contrary, reported that 16 participants reported increased frequency, duration of seizures, or episodes of status epilepticus; in two cases, doctors considered a plausible relationship with CBD treatment.[18] About 67% of patients experienced a 95% or greater reduction in seizure frequency in the first 2 weeks alone; however, only 50% (3/6) maintained the same efficacy throughout the study.[21]
Side effects
Out of the eight studies, all reported adverse effects related to CBD treatment, mostly mild-to-moderate effects that were temporary or reversed with adjustment to the CBD dose. The main adverse effect with the highest prevalence among patients was drowsiness, which was present in all eight studies analyzed, followed by diarrhea and loss of appetite. Other less frequently reported effects were irritability or behavioral problems, weight loss, nausea, vomiting, mood changes, insomnia, blurred vision, dry mouth, fever, agitation, and spasms. Other adverse effects were considered severe, being mentioned such as the elevation of liver enzymes, reported in 4/8 studies, where it was reversed by reducing CBD doses and, in one case, by removing the pharmacological interaction between CBD and valproic acid.
Quality of the studies included
Using CASP, the quality of the included articles was assessed by 11 questions in the case of cohort studies, nine questions for quasi-experimental studies, and eight questions in the case of analytical cross-sectional studies. Assigning one point (1) if the question was corresponding, 0.5 if it was unclear, and zero (0) if it was not corresponding. The quality of the studies indicated that the average score was 8.17 ± 1.61 for cohort studies, 7.33 ± 0.29 for quasi-experimental studies, and 7.0 ± 0.0 for analytical cross-sectional studies [Figure 2].
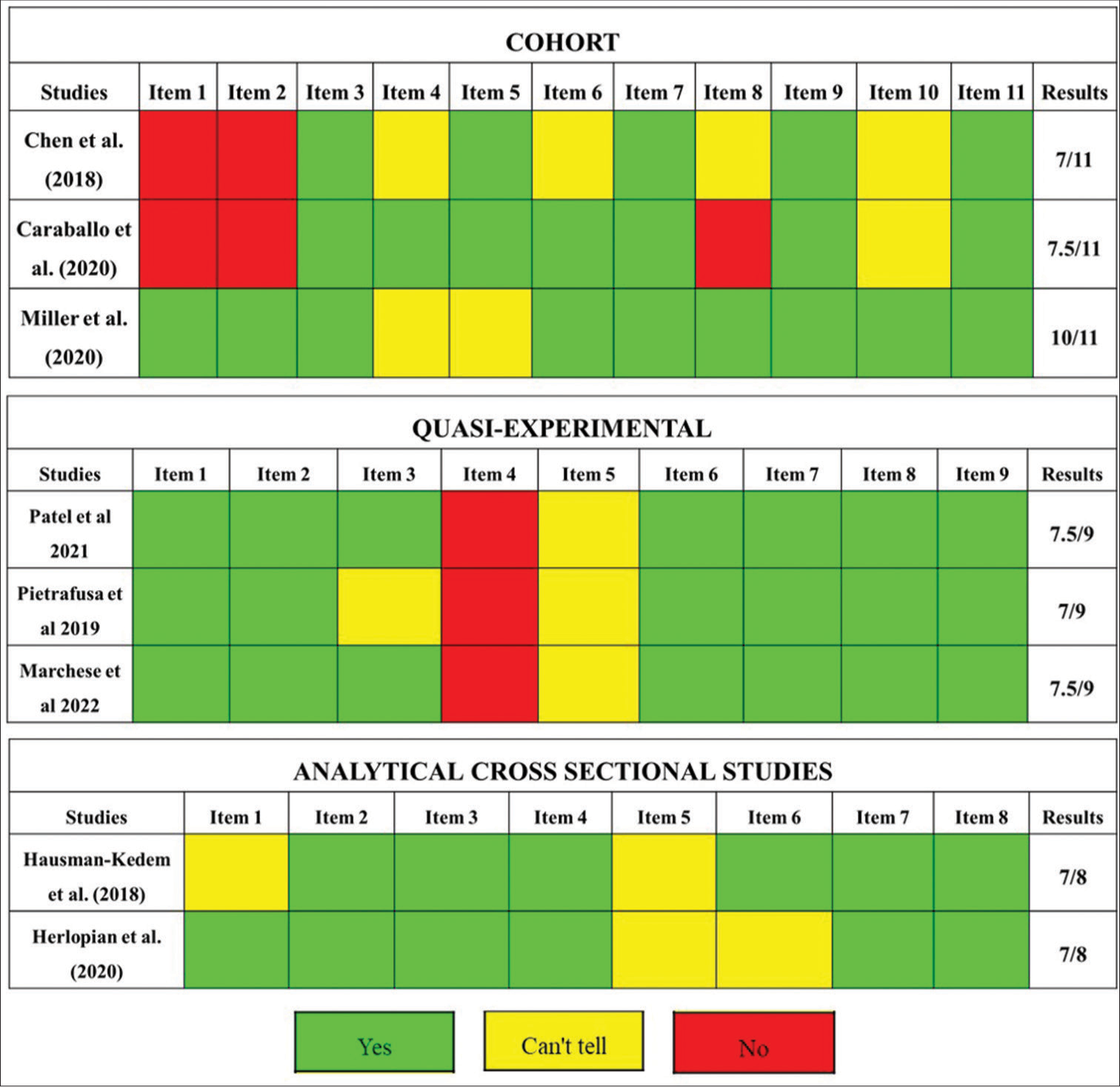
- Evaluation of quality of included studies by Joana Briggs Institute checklist. Green: 1 point, Yellow: 0.5 points, Red: No point. In order to evaluate the quality of the included studies, these were evaluated with items that allow to know their quality. Each item has a question. Each question answered correctly is equivalent to 1 point and is colored green, answered partially is equivalent to 0.5 point and is colored yellow, and not answered is equivalent to no point and is colored red.
DISCUSSION
Despite the existence of records proving the use of cannabis since ancient times, it is only in recent years that studies have been conducted demonstrating the efficacy and safety of the use of cannabis compounds in epilepsy in pediatric patients and young patients. In this review, multiple researchers mostly observed that CBD could positively impact seizures in patients. These models provide significant results to validate the efficacy of cannabinoids in the regulation of neurological processes involved in the regulation of GABA and glutamate neurotransmitters.
In the review of eight articles with pediatric and young populations, CBD was administered with purity between 24% and 98%, which showed improvements in tonic-clonic seizures of more than 50% and a complete reduction of seizures in patients, in agreement with the findings of Lazarini–Lopes et al., who indicated that CBD reduced the generalized tonic-clonic seizures dose-dependent of GEPR-3, as well as decreasing the severity and duration of limbic seizures of the brainstem in animal models at doses of 10 mg/kg.[24] It could be explained by some mechanisms in which the endocannabinoid system is directly associated with the brainstem and limbic neural networks, which could modulate the activity of GABA as well as the functioning of sodium and calcium channels induced by CBD, which can reduce neuronal excitability, prevent the propagation of convulsions, and finally generate a reduction in the frequency.[25]
Similarly, in epileptic encephalopathy, 51% of patients showed improvements in seizure reduction, 3% were seizure-free, and 3% showed improvements of <50% regardless of their convulsions, which is notably supported by Caraballo et al., where it has been shown that the use of CBD resulted in an 80% decrease in epileptic encephalopathy.[19] In the same way, in the approach to infantile syndromes of complex management such as Dravet syndrome and Lennox–Gastaut syndrome, some studies, such as GWPCARE1 Part B and GWPCARE2, addressed patients mostly under age, identifying an average decrease of 45–74% in the monthly frequency of seizures in a period of 12 weeks up to week 156. As for total seizures, the average reduction was 49–84%.[26]
In individuals affected by epileptic encephalopathy with SYNGAP1 gene expression, it has been observed that CBD induces an increase in the expression of the cation channel subfamily V member 1 protein. This channel plays a crucial role in the mechanisms underlying the excitatory/inhibitory imbalance associated with drug-resistant epilepsy. CBD has been shown to cause rapid activation and desensitization of the aforementioned member 1, leading to channel regulation.[27] This phenomenon has also been explored in the context of the SCN1A genes linked to Dravet syndrome, which encode the NaV1.1 and NaV1.2 sodium channels. These channels are highly expressed in the brains of patients with this epileptic pathology and are mainly affected by the loss of function due to stop codons, deletions, or single residue inactivating mutations in the SCN1A gene.[28] In this context, it has been identified that CBD has the ability to prevent the opening of NaV1.2 and NaV1.6 channels, inhibiting their electrical activity and thereby reducing the seizures associated with these syndromes.[29]
At the pediatric level, even though there are benefits in the consumption of CBD, doses higher than 50 mg/kg/day should be evaluated as they may act as triggers for side effects in children following the administration of cannabis. Most of the pediatric epilepsy studies reported high rates of these side effects, which tended to be mild, such as gastrointestinal symptoms reported in patients taking more than 15 mg/kg/day, increasing drug intolerance, and the likelihood of reports of diarrhea or related side effects (weight loss, nausea, and decreased appetite). Likewise, potential associations have been reported between the administration of CBD oils and the development of microscopic colitis.[30,31]
In addition, drowsiness and fatigue have been observed as a result of the interaction between clobazam and CBD. It is important to mention that clobazam was used simultaneously with CBD in some cases and that the patients were on this drug before the CBD treatment began; furthermore, in the majority of cases, clobazam was used in the majority of monitored patients because it is a drug with a common usage among the different types of refractory epilepsy and shows the reaction between the two drugs. The side effects are derived from the increase in the concentrations of the clobazam metabolite, N-desmethyl clobazam, through the inhibition of CYP2C19 by CBD, thus intensifying its effects.[29,32] Elevations in liver enzymes are noted, especially in children taking valproic acid, which is associated with worsening seizures and blood dyscrasias, with possible effects on cognitive development.[14] The interaction between valproic acid and CBD generates transient metabolic stress in the liver in patients who suspend treatment. On the other hand, evidence related to short-term memory impairment has been found as an established effect of acute cannabis intoxication, attributed to THC on hippocampal CB1 receptors.[30] THC and CB1 agonists reduce presynaptic neurotransmitter release, thereby altering long-term synaptic plasticity in the hippocampus. On the other hand, extensive research has indicated that CBD consumption could influence cognitive performance, which can be supported by randomized studies in which individuals exposed to CBD in a single dose (12.5 mg CBD) established better performance in verbal episodic memory but not in working memory.[33] However, other research has indicated that administration of 800 mg of CBD daily may improve working memory manipulation.[34]
CONCLUSION
The use of cannabis is a tool for refractory epilepsy when first-line therapies do not have the expected efficacy. The benefits of the crisis are clear, in the reduction and even elimination or blocking of seizures, impacting positively their quality of life such as sleep, behavior, and cognitive functions. There is great efficacy against different types of epileptic seizures, such as tonic, tonic-clonic, epileptic encephalopathy, focal seizures, and generalized seizures, making its use advisable for patients, without forgetting that more information is still required regarding its long-term use. As for the adverse effects, it can be noted that, despite being almost constant, these mainly appear due to the interaction of CBD with the medications used by these patients, although it is clear that none of these adverse effects turned out to be a reason not to stop the treatment that was presented during the different studies.
Acknowledgments
The authors thank GINUMED, Medicine, Rafael Núñez University Corporation, Cartagena D.T. y C.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The quality of life of children with epilepsy and the impact of the disease on the family functioning, public health. Int J Environ Res Public Health. 2022;19:2277.
- [CrossRef] [PubMed] [Google Scholar]
- Epilepsy and attention deficit hyperactivity disorder: Connection, chance, and challenges. Int J Mol Sci. 2023;24:5270.
- [CrossRef] [PubMed] [Google Scholar]
- An approach to neurometabolic epilepsy in children with an underlying neurometabolic disorder. Iran J Child Neurol. 2020;14:79-86.
- [Google Scholar]
- Approaches to refractory epilepsy. Ann Indian Acad Neurol. 2014;17(Suppl 1):S12-7.
- [CrossRef] [PubMed] [Google Scholar]
- The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia. 2018;59:2179-93.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic factors and the risk of drug-resistant epilepsy in young children with epilepsy and neurodevelopment disability: A prospective study and updated meta-analysis. Medicine (United States). 2021;100:E25277.
- [CrossRef] [PubMed] [Google Scholar]
- Developmental and epileptic encephalopathies: Recognition and approaches to care. Epileptic Disord. 2021;23:40-52.
- [CrossRef] [PubMed] [Google Scholar]
- Dravet syndrome: A systematic literature review of the illness burden. Epilepsia Open. 2023;8:1256-70.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabinomics: Application of metabolomics in cannabis (Cannabis sativa L.) Research and development. Front Plant Sci. 2020;11:554.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabis and Its secondary metabolites: Their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines (Basel). 2019;6:31.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological and therapeutic properties of cannabidiol for epilepsy. Drugs. 2019;79:1435-54.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging use of epidiolex (Cannabidiol) in epilepsy. J Pediatr Pharmacol Ther. 2020;25:485-99.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- [CrossRef] [PubMed] [Google Scholar]
- Purified cannabidiol for treatment of refractory epilepsies in pediatric patients with developmental and epileptic encephalopathy. Pediatr Drugs. 2019;21:283-90.
- [CrossRef] [PubMed] [Google Scholar]
- An open retrospective study of a standardized cannabidiol based-oil in treatment-resistant epilepsy. Cannabis Cannabinoid Res. 2022;7:199-206.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabidiol for treating drug-resistant epilepsy in children: The New South Wales experience. Med J Aust. 2018;209:217-21.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of cannabidiol in a prospective cohort of children with drug-resistant epileptic encephalopathy in Argentina. Seizure. 2020;80:75-80.
- [CrossRef] [Google Scholar]
- Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents-an observational, longitudinal study. Brain Dev. 2018;40:544-51.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabidiol in treatment of refractory epileptic spasms: An open-label study. Epilepsy Behav. 2020;106:106988.
- [CrossRef] [PubMed] [Google Scholar]
- Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: A randomized clinical trial. JAMA Neurol. 2020;77:613-21.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term safety and efficacy of add-on cannabidiol in patients with Lennox-Gastaut syndrome: Results of a long-term open-label extension trial. Epilepsia. 2021;62:2228-39.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabidiol attenuates generalized tonic-clonic and suppresses limbic seizures in the genetically epilepsy-prone rats (GEPR-3) strain. Pharmacol Rep. 2023;75:166-76.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabinoids in audiogenic seizures: From neuronal networks to future perspectives for epilepsy treatment. Front Behav Neurosci. 2021;15:611902.
- [CrossRef] [PubMed] [Google Scholar]
- Add-on cannabidiol in patients with Dravet syndrome: Results of a long-term open-label extension trial. Epilepsia. 2021;62:2505-17.
- [CrossRef] [PubMed] [Google Scholar]
- Forty years of sodium channels: Structure, function, pharmacology, and epilepsy. Neurochem Res. 2017;42:2495-504.
- [CrossRef] [PubMed] [Google Scholar]
- Highly purified cannabidiol for epilepsy treatment: A systematic review of epileptic conditions beyond Dravet syndrome and Lennox-Gastaut syndrome. CNS Drugs. 2021;35:265-81.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical pharmacology of cannabidiol in refractory epilepsy. Farm Hosp. 2020;44:222-9.
- [Google Scholar]
- Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-20.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabidiol oil-associated microscopic colitis. Cureus. 2020;12:e10528.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse events of cannabidiol use in patients with epilepsy: A systematic review and meta-analysis. JAMA Netw Open. 2023;6:E239126.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabidiol enhances verbal episodic memory in healthy young participants: A randomized clinical trial. J Psychiatr Res. 2021;143:327-33.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of four-week cannabidiol treatment on cognitive function: secondary outcomes from a randomised clinical trial for the treatment of cannabis use disorder. Psychopharmacology (Berl). 2023;240:337-46.
- [CrossRef] [PubMed] [Google Scholar]







