Translate this page into:
Upper Extremity Monoplegia following Prone Surrender Position for Spinal Surgery
Gazanfar Rahmathulla, MD Department of Neurosurgery, University of Florida College of Medicine Jacksonville. 653-1 8th St. W, Jacksonville, FL 32209 United States gazanfar.rahmathulla@jax.ufl.edu
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Secondary peripheral nerve injuries remain a significant perioperative problem due to patient positioning and contribute to reduced patient quality of life and exacerbated professional liability. Comorbidities and concomitant lesions can further elicit these injuries in patients undergoing spinal surgeries.
Case Presentation We report a case of a 70-year-old male polytrauma patient presenting with a left first-rib fracture and an adjacent hematoma around the brachial plexus without preoperative deficits. Subsequent to a lumbar spinal fusion in the prone position, he developed a postoperative left upper extremity monoplegia. The postoperative magnetic resonance imaging revealed an enhanced asymmetric signal in the trunks and cords of the left brachial plexus. He progressively improved with rehabilitation, a year after the initial presentation, with a residual wrist drop.
Conclusions Pan brachial plexus monoplegia, following spine surgery, is rare and under-reported pathology. To minimize the occurrence of this rare morbidity, appropriate considerations in preoperative evaluation and counseling, patient positioning, intraoperative anesthetic, and electrophysiological monitoring should be performed. We emphasize an unreported risk factor in polytrauma patients, predisposing this rare injury that is associated with prone spinal surgery positioning, SEPs being an extremely sensitive test intraoperatively and highlight the importance of counseling patients and families to the possibility of this rare occurrence.
Keywords
positioning nerve injury
peripheral nerve
brachial plexus palsy
brachial plexus monoplegia
Introduction
Peripheral nerve injuries secondary to intraoperative patient positioning are a significant perioperative problem contributing to patient injury and increased professional liability.1 2 The prone surrender position has been associated with upper extremity neuropraxia, likely due to mechanical factors of stretch and compression of the affected nerves.2 However, there are no reports of complete brachial plexopathy in neurologically intact patients.
We present a rare postoperative near-complete brachial plexopathy in an elderly polytrauma patient following spinal surgery in the prone position and discuss the importance of positioning, anesthetic considerations, and the role of neuromonitoring in these cases.
Case Report
Case Presentation
A 70-year-old man presented with polytrauma following a high-speed motor vehicle collision in hemorrhagic shock. Examination revealed the presence of a “seat belt sign” (contusions and abrasions in the thoracic and abdominal area of a restrained passenger due to wearing a seat belt when involved in a motor vehicle crash) noted to the left subclavian area and chest area with a skin tear to the left clavicle. Trauma exams revealed hepatic and intestine injuries that necessitated an emergent exploratory laparotomy. The patient had left first and fourth rib fractures with an adjacent hematoma around the brachial plexus, likely due to the dissection of the left subclavian artery and proximal occlusion of the left vertebral artery. He underwent several operations to repair his systemic injuries.
Computed tomography imaging revealed a three-column flexion injury through the L5-S1 space with L5 pedicle lamina fracture involving the inferior L5 endplate and superior S1 endplate fracture. His neurological examination was unremarkable except for 4/5 strength in the extensor hallucis longus.
Magnetic resonance imaging (MRI) of the lumbar spine performed 3 weeks into his hospital course revealed a left L5 pedicular fracture extending into the lamina and L5-S1 articular facets, consistent with an AO type B1 injury with PLC disruption; the decision was thus made to perform an instrumented fusion for stabilization of this injury.
The patient was placed in prone with the arms in the prone surrender (superman) position with neuromonitoring of the motor evoked potentials (MEPs), somatosensory evoked potentials (SSEPs), and electromyography (EMG). SSEPs and EMG recordings were not reliable in the upper extremities but were recordable and remained stable compared with a pre-positioning baseline. During the case, left upper SSEPs disappeared, anesthesiology repositioned the extremity, and other factors evaluated making sure the patient remained euthermic, maintaining appropriate mean arterial pressures. MEPs were consistently present in all extremities, no EMG changes were recorded through the case. Nerve conduction studies were not performed intraoperatively but were planned during the postoperative follow-up at 3 months.
On postoperative day 1, he had a painless left upper monoparesis involving upper and lower trunks of the brachial plexus, the lower being worse than the upper. He also had wrist drop with biceps, shoulder abduction, and tricep strength significantly diminished (muscular strength in deltoid 2/5, biceps 1/5, triceps 3/5, wrist extension 1/5, interosseous 2/5, and grip 2/5). He was areflexic on the left upper extremity with diminished tone and complained of diminished sensation and patchy loss in the tips of his fingers. An MRI showed asymmetric signal increase in the left brachial plexus trunks, roots and cords in the region of the left first rib displaced fracture (Figs. 1 and 2). Surrounding tissue edema was also extensive. Neurological examination at discharge (1 month postoperatively) demonstrated a marginal improvement in proximal strength. At subsequent 3-month of follow-up with rehabilitation, the left wrist drop persisted though biceps and triceps weakness were markedly improved, and at 15 months, he had complete proximal improvement with a grip and finger extension of 3 to 4/5. The patient regained near total recovery of his left upper extremity and with the progressive improvement, we did not feel further nerve conduction studies would change our management plan.
-
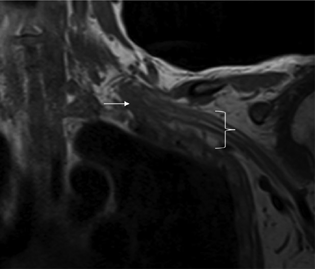
Fig. 1 Coronal T1 MRI demonstrates soft tissue injury and hematoma (white arrow) involving the left brachial plexus in the lower trunk region. Note the distal brachial plexus with a more normal appearance (white bracket). MRI, magnetic resonance imaging.
Fig. 1 Coronal T1 MRI demonstrates soft tissue injury and hematoma (white arrow) involving the left brachial plexus in the lower trunk region. Note the distal brachial plexus with a more normal appearance (white bracket). MRI, magnetic resonance imaging.
-
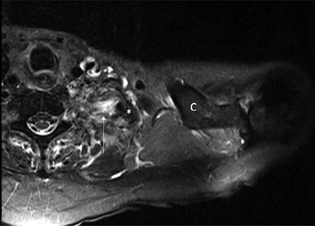
Fig. 2 Axial STIR (arrow) MRI demonstrates injury to the left brachial plexus medial to a fractured first rib fragment (*). Clavicle (C). MRI, magnetic resonance imaging.
Fig. 2 Axial STIR (arrow) MRI demonstrates injury to the left brachial plexus medial to a fractured first rib fragment (*). Clavicle (C). MRI, magnetic resonance imaging.
Discussion
Despite efforts to prevent compression injuries from positioning and protect the peripheral nerves during long-lasting surgery, 0.14% of peripheral nerve injuries result from improper operative placements, with peripheral neuropathy presenting most commonly with reports of injury as high as 40%.1 Posterior spinal surgery is the preferred approach for a wide-range of procedures including instrumentation and fusion, for spinal pathology including tumors of the spine, fractures, infections, and degenerative conditions. Positioning requires the patients arms be placed either by the patients side in a papoose or in the prone surrender “superman” position by placing the shoulders in abduction, no higher than the operating table, and placing the elbows at a right angle2 3. One of the major complications incurred during posterior approaches to the spine are perioperative peripheral nerve injury (PPNI),4 being brachial, radial, and ulnar neuropathies some of the most common injuries, with a 3.3% estimated incidence rate.4 PPNI can be also precipitated by pre-existent peripheral neuropathies that are most commonly observed in patients over 60 years,5 with chronic conditions (as presented in this case report), tobacco users, with high body mass index, increased age, and male sex.6 7 Other rare idiopathic and inflammatory factors likely play a significant role in its presentation.8 9 However, the leading cause of PPNI development is positioning during long-lasting surgeries in the prone steep Trendelenburg position, with shoulder or wrist traction.1
The proximity of the brachial plexus to mobile bony structures increases the risk of damage when stretched or compressed secondary to improper surgical positioning.10 Moreover, prone positioning can result in perioperative visual loss, skin pressure necrosis (36%), displacement of catheters/venous lines (0.06%), and cannulas' extubation (1.2%).6 7 11 12
The advantages of such position during occipital cranial approaches and spine surgeries ultimately overcome the risk associated with other complications. The main advantages include (1) ability to easily access the neural structures including the spinal cord and nerve roots, (2) significantly decreased morbidity and mortality compared with approached from the thorax and abdomen, (3) improved surgical field visualization, and (4) maintenance of the surgical equipment out of the surgical field. Alternatively, the limitations described include (1) pressure ulcers that arise when bony structures are compressed against pads and holders,11 12 13 (2) circulatory changes with a reduction of 20% in the cardiac index due to venous cava compression and reduced ventricular filling,12 13 and (3) airway compromise secondary to ventilator displacements driven by the patient facing down.11
Mechanical factors predisposing to PPNI occurs when a nerve is stretched, transect, or compressed.12 13 Nerve's mechanical stress more than 5 to 15% of regular length and hypoperfusion result in ischemia, edema, and axonal damage, causing conductance dysfunction.12 13 Cadaveric studies12 suggest that brachial plexopathy in the prone position is the most commonly identified risk factor resulting from arm abductions greater than 90 degrees.13 Similarly, extension and external rotation of the arms, ipsilateral rotation, lateral flexion of the neck, and shoulder braces are amongst the most commonly associated risk factors of brachial plexus-prone-related injuries described by some scholars.3 Similarly, anesthesia can cause nerve damage via ischemia due to vasoconstriction or by chemical imbalance increasing internal and external nerve pressure.4 Compressing hematomas, can rarely cause gradual brachial plexus palsies, especially after jugular vein catheterization, axillary artery injuries, or blunt trauma in the shoulder.2
Intraoperative neuromonitoring studies are an essential tool to detect nerve damage and prevent injuries. The SSEPs appear to be a sensitive indicator of the likelihood of nerves stretching beyond normal thresholds and may serve as a potential early indicator for peripheral nerve ischemia. However, the false-negative rate of SSEPs for detecting postoperative nerve injury has been estimated at 43%.14 In our case, anesthesiology verified positioning multiple times and repositioned the SSEPs in the left upper extremity, when signal was lost. MEPs were present throughout the case and did not appear sensitive or specific in the early capture of peripheral nerve injury during surgery. Nerve conduction studies were not performed as no clinical benefit would be offered to the patient.
Additionally, repositioning, for example, by tucking the arms at the patient's side,7 use of protective padding, and avoidance of tourniquets and airway' retractors can help us to decrease injuries1 2 Finally, the ASA task force has published a series of precautionary measures to consider.15 Notwithstanding the volatility of anesthetic agents complicating neuromonitoring signals, the anesthesiologist, along with the rest of the operating team, are encouraged to optimize factors that mitigate complications: intraoperative MAPs > 80 mm Hg avoid prolonged hypotension and optimize anesthesia drug protocols to enable sensitive and specific SSEPs monitoring and recording.14
In our case, the patient's significant trauma-related comorbidities, local hypoperfusion secondary to the rib fracture, hematoma, and edema may have synergistically contributed to the loss of reserve in the plexus, facilitating the brachial plexopathy, even though all precautions and appropriate positioning changes during the procedure are performed by the anesthesiology team.
Conclusions
Surgical positioning has evolved and is extremely safe, given the technological advances and the ability to monitor numerous patient parameters. Precautions to prevent iatrogenic injury during surgical position are routinely practiced. Elderly polytrauma patients with comorbidities are at an increased risk for positional peripheral nerve injury during surgical procedures. Electrophysiological monitoring with somatosensory potentials remains a sensitive means to detect peripheral nerve compression, enabling proactive position adjustments during surgery. Appropriate preoperative evaluation of patient pathology, image findings, and surgical position planning taking into account adjacent tissue trauma, adequate counseling, and the use of intraoperative neurophysiological monitoring, along with collaborative teamwork with anesthesiology are essential in mitigating and reducing the risk of these rare injuries.
Conflict of Interest
None declared.
Funding None.
References
- Complications associated with prone positioning in elective spinal surgery. World J Orthop. 2015;6(3):351-359.
- [Google Scholar]
- Perioperative peripheral nerve injury after general anesthesia: a qualitative systematic review. Anesth Analg. 2018;127(1):134-143.
- [Google Scholar]
- Perioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-year period at a single institution. Anesthesiology. 2009;111(3):490-497.
- [Google Scholar]
- Kyphosis - A risk factor for positioning brachial plexopathy during spinal surgeries. Acta Orthop Traumatol Turc. 2019;53(3):199-202.
- [Google Scholar]
- Brachial plexus injury following spinal surgery. J Neurosurg Spine. 2010;13(4):552-558.
- [Google Scholar]
- Prone positioning of patients with acute respiratory distress syndrome: a systematic review. Am J Crit Care. 1999;8(6):397-405.
- [Google Scholar]
- Predictive factors for hypotension associated with supine-to-prone positional change in patients undergoing spine surgery. J Neurosurg Anesthesiol. 2020;32(2):140-146.
- [Google Scholar]
- Blood flow of peripheral nerve effects of dissection, stretching and compression. J Hand Surg [Br]. 1986;11(1):10-14.
- [Google Scholar]
- Somatosensory evoked potential monitoring of lumbar pedicle screw placement for in situ posterior spinal fusion. Spine J. 2003;3(5):370-376.
- [Google Scholar]
- Practice advisory for the prevention of perioperative peripheral neuropathies: a report by the American Society of Anesthesiologists Task Force on Prevention of Perioperative Peripheral Neuropathies. Anesthesiology. 2000;92(4):1168-1182.
- [Google Scholar]






