Translate this page into:
Unique presentation of occipital condyle giant cell tumor as occipital condyle syndrome – A review
*Corresponding author: Ganesh Divakar, Department of Neurosurgery, Sree Chitra Tirunal Institute of Medical Sciences and Technology, Thiruvananthapuram, Kerala, India. drganeshdivakar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Matham G, Divakar G, Deepti AN, Thomas B, Easwer HV, Kumar K. Unique presentation of occipital condyle giant cell tumor as occipital condyle syndrome – A review. J Neurosci Rural Pract 2023;14:3-6.
Abstract
Giant cell tumors (GCTs) of the skull are rare and only a few case series with limited number of cases have been reported till date. In the cranium, GCT usually occurs in the sphenoid and temporal bone, occipital condyle GCTs are very rare. We report a rare presentation of GCT of the occipital condyle manifested as occipital condyle syndrome. Despite gross total resection, they can recur aggressively; the presence of cortical breach might be an indicator of aggressiveness prompting early post-operative imaging and adjuvant therapy.
Keywords
Occipital condyle
Giant cell tumor
Occipital condyle syndrome
INTRODUCTION
Giant cell tumors (GCTs) are locally aggressive, destructive bony lesions, usually found in the epiphysis of long bones.[1] GCTs of the skull are rare, with a predilection for sphenoid and temporal bone;[2] occipital condyle GCTs are extremely rare. Occipital condyle syndrome (OCS) is characterized by continuous unilateral occipital headache, associated with ipsilateral hypoglossal nerve paralysis. Although there are very few reported cases of GCTs involving the occipital condyle, its presentation solely as OCS has never been reported.
CASE SUMMARY
A 22-year-old girl presented to us with 3 months duration of insidious onset gradually progressive throbbing type of the right suboccipital headache radiating to the neck and aggravated with neck movements. After a few days, she noticed slurring of speech and deviation of tongue to the right side. Clinical examination was unremarkable except for the right lower motor neuron (LMN) hypoglossal palsy. Computed tomography of the skull showed an expansile lytic lesion involving the right occipital condyle with thinning and breach of the cortex. Magnetic resonance imaging (MRI) showed 2 × 2.4 × 1.6 cm intensely enhancing homogeneous mass in the right occipital condyle, with restricted diffusion, encroaching onto the right hypoglossal canal and jugular foramen, and suggesting an aggressive primary bony lesion. There was no soft-tissue enhancement surrounding the lesion. She underwent far lateral approach and gross total decompression of the tumor, along with occipitoaxial instrumented stabilization in view of potential instability caused by the tumor involving the articular surface of the occipital condyle. Histopathology revealed a tumor composed of mononuclear round cells displaying moderate pleomorphism and few typical mitotic figures with several uniformly distributed osteoclast-like giant cells with reactive bone formation rendering a diagnosis of giant cell tumor [Figure 1a-h].
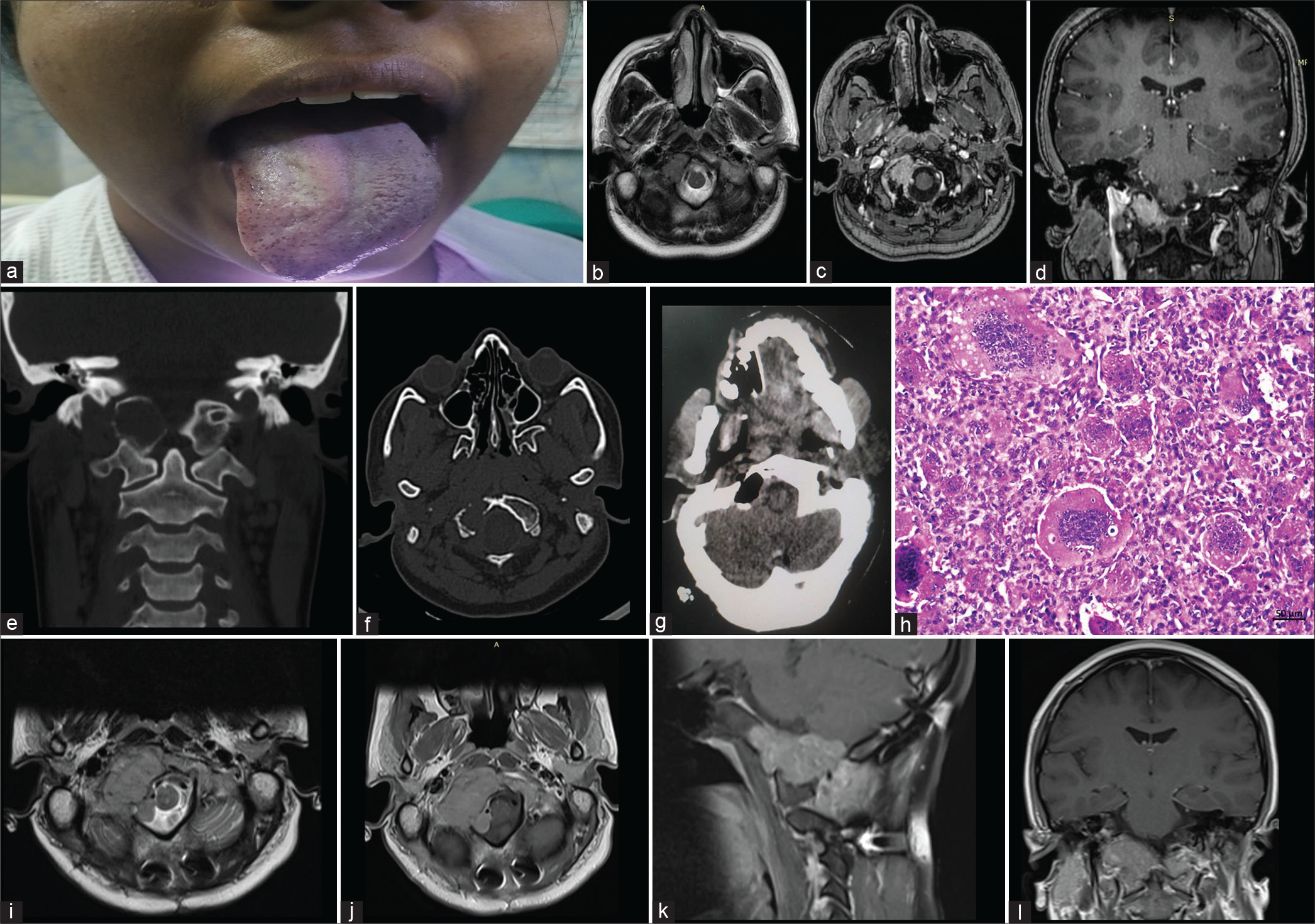
- (a) Clinical picture showing right LMN hypoglossal palsy, (b) pre-operative magnetic resonance imaging (MRI) T2 axial images showing hypointense right occipital condylar lesion, (c and d) post-contrast images showing enhancing mass in the right occipital condyle (axial and coronal respectively), (e and f) coronal and axial computed tomography image showing the lesion, (g) post-operative cavity and excision of the right occipital condyle lesion, and (h) tumor comprising mononuclear stromal cells interspersed with osteoclast-like giant cells (hematoxylin and eosin, original magnification ×200 and scale bar 50 μm). (i) Axial T2 MRI showing large hypointense recurrence in the right occipital condylar region and (j-l) post-contrast MRI showing moderately enhancing large recurrent lesion in axial, sagittal, and coronal planes, respectively.
Postoperatively, the patient had good relief of headache with static neurological deficit as preoperatively.
At 3 months follow-up, the patient remained headache free, but a routine follow-up MRI showed evidence of large recurrent lesion extending to the clivus. The patient underwent adjuvant therapy with denosumab followed [Figure 1i-l] by radiation therapy. Follow-up imaging at 1 year showed stable residual lesion, the patient remained asymptomatic except for residual right hypoglossal palsy.
DISCUSSION
GCTs are primary bone tumors that develop through endochondral ossification. Hence, they are frequently seen in the long bone epiphysis, constituting 5% of the primary bone tumors.[3] GCT in cranium and spine constitutes only <1%,[4] sphenoid being the most common, followed by petrous temporal, parietal, and frontal bones. This variation is due to the fact that sphenoid and temporal bone develops through endochondral ossification. Occipital GCTs are extremely rare, with only 11 case reports available in the literature [Table 1].[19-21] Most authors have reported cases presenting with occipital headache and no physical findings.[1,5-7] Opitz et al. have reported an occipital GCT in a case of von Recklinghausen’s disease.[5] Kajiwara et al. presented a case of occipital GCT with neck pain, dysphoria, and dysphagia, who was treated with denosumab. Motomochi[2] reported a case involving the occipital condyle, with multiple cranial nerve palsies. Clinical data were not available in four cases. Our case of occipital condyle GCT has presented solely with OCS.
| Study | Age/sex | Clinical features | Region | Extent of resection | Adjuvant therapy | Recurrence |
|---|---|---|---|---|---|---|
| Troell[21] 1930 | 20/M | NA | Occipital | PR | No | NA |
| Arseni et al.[19]1975 | 8/F | NA | Occipital | STR | No | NA |
| Motomochi et al.[2] 1985 | 53/M | Headache, dysphagia and dysarthria, 9,10,11,and 12 CN palsy | Occipital bone, condyle, hypoglossal canal, and clivus | STR | Yes (RT) | No (26 months) |
| Bertoni et al.[3] 1992 | 58/F | NA | Occipital | STR | No | Yes (died after 1.5 years) |
| 24/F | NA | Occipital bone with petrous and sphenoid bone | STR | Yes (RT) | No (7 years) |
|
| Optiz et al.[5] 1996 | NA | NA | Occipital condyle | NA | NA | NA |
| Lu et al.[22] 2011 | 19/F | Headache and vomiting | Left occipital bone with petromastoid and temporal bone | GTR | No | No (1 year) |
| Zhang et al.[7] 2013 | 19/F | Headache and vomiting | Occipital | GTR | No | No (31 months) |
| Harris et al.[6] 2004 | 24/F | Headache, tenderness, external swelling | Occipital bone, along with dural involvement | GTR | No | NA |
| Gonca hanedan uslu[1] 2014 |
22/F | Neck pain, headache | Occipital bone with dural sinus involvement | STR | Yes (RT) | No (20 months) |
| Kajiwara and Takeshi[20] 2019 |
56/M | Right neck pain, dysphoria, and dysphagia | Right occipital bone with condyle | Biopsy | Denosumab | No (5 years) |
| Our case | 22/F | Right occipital headache, right hypoglossal palsy | Occipital condyle | GTR | Yes (RT and denosumab) | Yes (1 year) |
CN, cranial nerves; F, female; GCT, giant cell tumor; GTR, gross total resection; M, male; NA, data not available; PR, partial resection; RT, radio therapy; STR, subtotal resection.
OCS, first described by Greenberg and Deck,[8] is characterized by continuous occipital headache, with radiating neck pain aggravated by neck movements away from the side of pain and relieved by rotation toward the side of pain.[9] Some patients may also have occipital tenderness. Usually, hypoglossal palsy manifests after the patient gets a headache. Metastases and primary skull base tumors are commonly associated with OCS in up to half of the patients. Metastases usually originate from the breast, lung, thyroid, gastrointestinal tract, pharyngeal tumors, lymphomas, and prostate.[10]
MRI and CT are the imaging modalities of choice. However, radiological features alone are not sufficient to diagnose GCT. It is challenging to differentiate skull base GCTs from other benign lesions such as brown tumors of hyperparathyroidism, giant cell reparative granuloma, cherubism, and aneurysmal bone cyst. Therefore, to avoid confusion, clinical, radiological, and pathological findings are to be correlated for the definite diagnosis of skull base GCT.[11]
Campanacci and Baldini had designed a staging system for GCT, with Stage I tumors limited to bone but without cortical involvement; Stage II tumors involving the cortex but without cortical breach; and Stage III tumors that breach the cortex and extend into soft tissues.[12]
The treatment of choice for GCTs is en bloc resection of the tumor whenever feasible.[13] However, skull base GCTs are often not amenable to complete resection due to the proximity to critical neurovascular structures. Good local tumor control can be achieved by gross total resection. Recurrence rates are as high as 40–60% with subtotal resections.[14] As most of these data are available from surgical series of long bone GCTs, it is doubtful whether this can be extrapolated to similar lesions of the skull base. Moreover, due to rarity of these cases, an optimal treatment strategy for skull base GCTs has not been described in the literature.
Histological grading is a poor predictor of clinical outcome. The radiological staging system by Campanacci and Baldini[11] neither delivers reliable prognostic value regarding recurrence rates or functional outcomes nor helps in deciding on the management options. Although some authors have reported Campanacci and Baldini Grade II/ III tumor excision and soft-tissue involvement as factors underlying increased recurrence rates, convincing evidence for the same could not be established.[15,16] We assume that, in our case, cortical destruction is the probable underlying cause for this early aggressive recurrence despite the tumor being relatively small in size.
Adjuvant therapy can reduce the chances of recurrence. Furthermore, many forms of radiation therapy and various chemotherapeutic agents were investigated in patients with unresectable tumors. Radiation therapy as a single agent was able to achieve control rates of up to 70–80%, but in the case of recurrent tumors, it was of limited response.[1] There were concerns of malignant transformations with radiotherapy but with newer highly conformal techniques of radiation, this seems to be insignificant.
Many chemotherapeutic agents such as doxorubicin, cisplatin, methotrexate, zoledronate, and raloxifene[17] have been tried. New target-based drugs are developed as the pathophysiology of GCT has been studied well. The neoplastic component of GCT is the stromal cell which overexpresses receptor activator of nuclear factor κB ligand (RANKL), while the osteoclast-like giant cells express RANK. RANKL is a member of the TNF family of molecules which bind to the RANK receptor. Denosumab is a human-derived monoclonal antibody against RANKL, which selectively binds human RANKL and inhibits osteoclast differentiation and osteoclast-mediated bone resorption. Results of a multicentric Phase II trial[18] regarding the usage of denosumab for the primary treatment as well as an adjunct in recurrent GCTs showed favorable risk-to-benefit ratio. Almost 99% of patients had a clinical benefit in either complete/partial reduction or non-progression. Denosumab (120 mg) is usually administered subcutaneously once every 4 weeks with additional loading doses on the 8th and 15th days. Three percentage of patients developed osteonecrosis of the jaw on prolonged denosumab treatment. Further studies are required to identify the optimal duration and dosage for minimizing complications.
CONCLUSION
OCS may be the sole manifestation of GCTs of the occipital condyle, which is an extremely rare site for these locally aggressive lesions. A high index of suspicion and early imaging is necessary to detect these lesions before they produce cortical breach and encroach adjacent critical neurovascular structures. From our own experience and available literature data, we feel that cortical breach is a predictor of early recurrence in such lesions, despite gross total removal. However, despite large lesion size, adjuvant denosumab and radiation therapy may be helpful in maintaining symptomatic quiescence, especially in regions, where en bloc resection is not feasible. Further, long-term follow-up data from a sizeable number of such patients are required to develop a standard of care in skull base GCTs.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Giant cell tumor of the occipital bone: A case report and review of the literature. Oncol Lett. 2014;8:151-4.
- [CrossRef] [PubMed] [Google Scholar]
- From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: Radiologic-pathologic correlation. Radiographics. 2001;21:1283-309.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of the occipital bone in a case of von Recklinghausen neurofibromatosis. Clin Neuropathol. 1996;15:226-30.
- [Google Scholar]
- Giant cell tumor of the skull: A case report and review of the literature. Surg Neurol. 2004;61:274-7.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumors of the skull: A series of 18 cases and review of the literature. J Neurooncol. 2013;115:437-4.
- [CrossRef] [PubMed] [Google Scholar]
- Metastasis to the base of the skull: Clinical findings in 43 patients. Neurology. 1981;31:530-7.
- [CrossRef] [PubMed] [Google Scholar]
- Occipital condyle syndrome as an initial presentation of lung cancer: A case report. Acta Neurol Taiwan. 2015;24:11-4.
- [Google Scholar]
- Occipital condyle syndrome caused by isolated bone metastases from thyroid cancer. Indian J Nucl Med. 2019;34:48-50.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of the skull. Radiographics. 1998;18:1295-302.
- [CrossRef] [PubMed] [Google Scholar]
- Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106-14.
- [CrossRef] [PubMed] [Google Scholar]
- RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol. 2012;24:397-403.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of the skull: Review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2016;77:239-46.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of bone: Treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134:969-78.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical considerations of cutaneous nerves of scalp for an effective anesthetic blockade for procedures on the scalp. J Neurosci Rural Pract. 2023;14:62-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clival giant cell tumor-a rare case report and review of literature with respect to current line of management. Asian J Neurosurg. 2017;12:78-81.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab in patients with giant-cell tumour of bone: A multicentre, open label, phase 2 study. Lancet Oncol. 2019;20:1719-29.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumors of the calvaria. J Neurosurg. 1975;42:535-40.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor in the skull base bone treated with anti-rankl inhibitor. Neurooncol Adv. 2019;1:ii38.
- [CrossRef] [Google Scholar]
- Zwei fälle von riesenzellentumor in Knochen beobachtet 3.5 bezw, 18 Jahre. Acta Chir Scand. 1930;67:906-13.
- [Google Scholar]
- Giant cell tumour of the posterior cranial fossa: A case report. Br J Radiol. 2011;84:e208-11.
- [CrossRef] [PubMed] [Google Scholar]






