Translate this page into:
Tumefactive perivascular space of the anterior temporal lobe
*Corresponding author: Amit Agarwal, Department of Radiology, University Texas Southwestern, Dallas, Texas, United States. amitmamc@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Agarwal A, Vibhute P. Tumefactive perivascular space of the anterior temporal lobe. J Neurosci Rural Pract. 2024;15:150-2. doi: 10.25259/JNRP_343_2023
Dear Editor,
Perivascular spaces (PVSs) are interstitial fluid-filled spaces lined by pia mater surrounding the penetrating arterioles for a variable distance, classically seen in the basal ganglia. “Tumefactive” PVS is rare entities where there is abnormal enlargement and complex configuration of PVS, usually seen in the mesencephalothalamic region, as clusters of uniform or variable-sized cysts, oriented along the perforating arterioles. Giant PVS is lesions, which are larger than 1.5 cm in size, most commonly seen in the midbrain region with frequent mass effect and obstructive symptoms including hydrocephalus. Gliosis and white matter T2/fluid-attenuated inversion recovery hyperintensities may occasionally be seen along these lesions; however, enhancement is invariably absent demarcating these from cystic neoplasms.[1] This demarcation can however be challenging at times, especially when perilesional edema is present with the differential diagnosis including enlarged PVSs, neuroepithelial cysts, and cystic neoplasms. The pathophysiology and clinical significance of these enlarged PVS have been poorly understood. The recent discovery of the brain glymphatic system has, however, provided a revolutionary perspective to understand the etiology of these lesions. This is a hot research topic these days, and it is now generally accepted that the PVS performs the same function as the lymphatic system in other parts of the body. This plays a significant role in the removal of metabolic waste and the preservation of homeostatic fluid circulation in the brain.[2] The mechanism of enlargement of the PVS is related to altered cerebral spinal fluid (CSF) or interstitial fluid dynamics including increased CSF pulsations or obstruction to the outflow secondary to amyloid vascular disease. Studies have reported an association of focal or diffuse enlargement of PVS with a wide range of metabolic conditions like mucopolysaccharidoses and with neurodegenerative conditions such as Alzheimer dementia and cerebral amyloid angiopathy.[3]
Anterior temporal lobe PVS is recently recognized special variant of “tumefactive” PVS, which mimics cystic tumors due to the presence of surrounding vasogenic edema in the majority (80%) of cases. These can be seen across a wide range of age groups ranging from the second to the ninth decade. These are also known as opercular PVSs as few cases of these entities have been described in the frontal operculum.[4] These are associated with a vascular branch of the middle cerebral artery (MCA) and the presence of perilesional edema makes it a unique variant of PVS. There are different theories regarding the cause of perilesional edema/signal change including the idea that compression of the adjacent parenchymal vessels by the enlarged PVS causes ischemic changes in the surrounding white matter. The extent of perilesional edema can range from mild to severe and can spontaneously increase or decrease over time. The cyst however remains stable, follows CSF signal on all sequences with invariable absence of contrast enhancement. These are characteristically seen along the anterior temporal pole and are associated with MCA branches with focal cortical thinning and/or distortion noted in vast majority of cases [Figure 1]. The presence of adjacent smaller PVS and a contiguous CSF intensity tract can usually be seen, especially if high-resolution T2-weighted magnetic resonance imaging (MRI) is performed.[3,4] Other imaging features, which are helpful in characterizing these structures, include their orientation and shape, which are frequently oblong or fusiform in morphology due to their association and extension along the course of a penetrating vessel. These are clinically occult and are usually detected incidentally on CT or MRI performed for some other indication. Although an increase in edema on sequential imaging may make it concerning for the radiologist and the clinician, the absence of any enhancement and the classic imaging features should serve as assuring features. Enhancement has never been reported in these lesions and change in the size of the cyst or the presence of enhancement should make one doubt the diagnosis. Confident diagnosis by the radiologist can help avoid unnecessary surgeries. Although imaging surveillance is reasonable, no treatment is required.[3,5] Given the limited knowledge about this entity, few cases have been misdiagnosed as cystic neoplasm and undergone biopsy or surgical resection. Histopathology in these cases has revealed penetrating vessels passing along pial-lined canals from the subarachnoid space to dilated PVSs. Reduction in myelin density and reactive astrocytic gliosis is also seen along these PVS. Dilated PVSs at other locations are known to spontaneously regress in some instances. However, given the recent identification of the anterior temporal PVS, no dedicated studies have focused on the long-term course of these entities. In the coming years, we anticipate that there will be a much better understanding of the glymphatic system and studies with larger sample sizes and longer follow-up of anterior temporal PVS will help us better understand this entity.
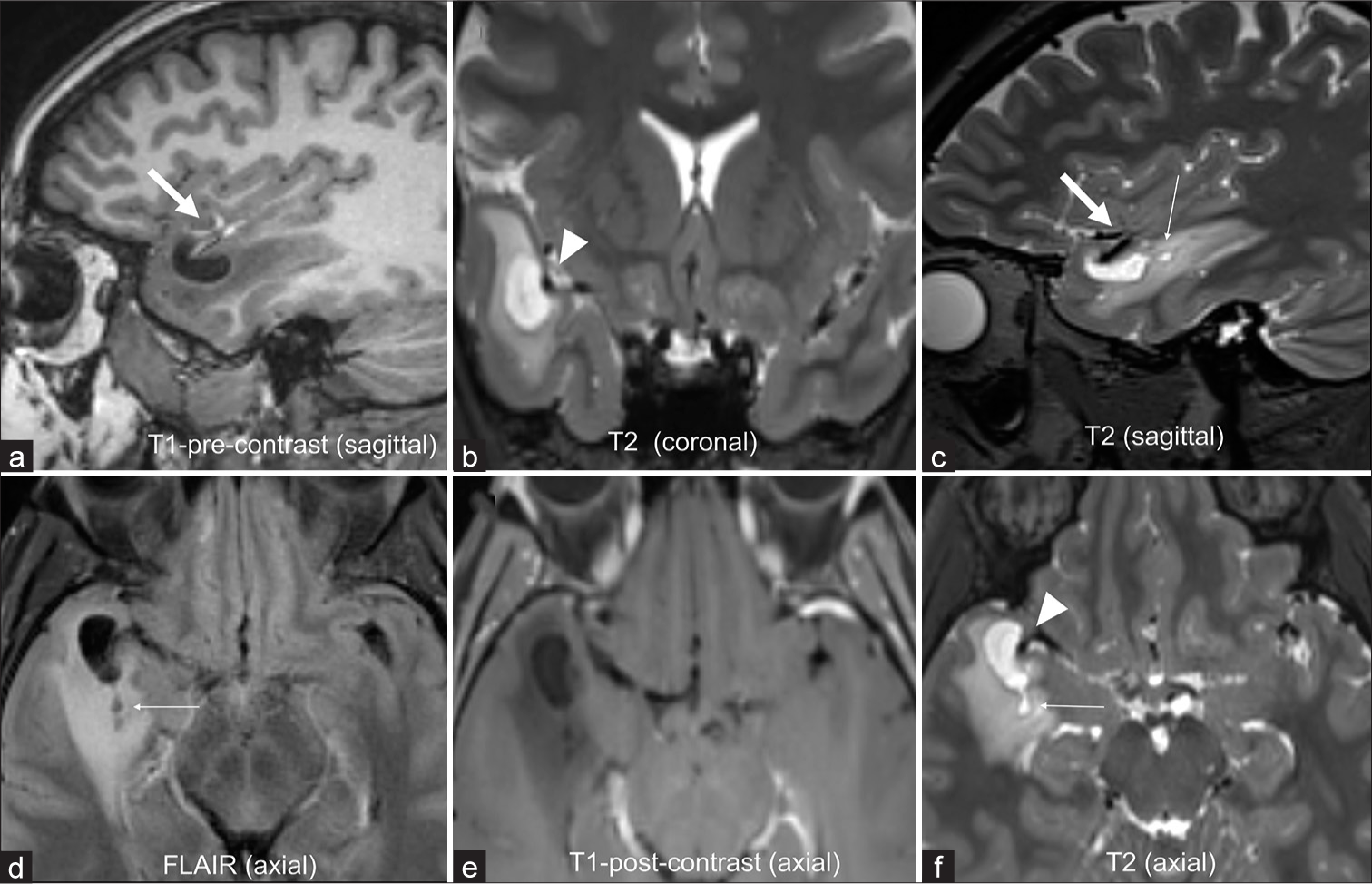
- A 34-year-old with an incidentally found brain “neoplasm” on emergency room head computed tomography. (a-f) Magnetic resonance imaging reveals a cystic lesion in the right anterior temporal pole following cerebral spinal fluid signal on all sequences, (a) T1-weighted and (b) T2-weighted (c,d) with moderate surrounding vasogenic edema. (e) No enhancement noted on the post-contrast axial image. (c, d, and f) Smaller cysts are noted posterior to the dominant lesion in a radial pattern (thin arrows). (a and c) Lesion is in immediate proximity to the middle cerebral artery cortical branch (thick arrows a and c) with focal cortical thinning, (b and f) best delineated on the T2- weighted images (arrowheads). Yearly imaging surveillance showed stable size of the cyst and surrounding edema over past three years and the patient remains asymptomatic.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Perivascular spaces, glymphatic system and MR. Front Neurol. 2022;13:844938.
- [CrossRef] [PubMed] [Google Scholar]
- Subcortical cystic lesions within the anterior superior temporal gyrus: A newly recognized characteristic location for dilated perivascular spaces. AJNR Am J Neuroradiol. 2014;35:317-22.
- [CrossRef] [PubMed] [Google Scholar]
- Opercular perivascular cysts: A proposed new subtype of dilated perivascular spaces. Eur J Radiol. 2020;124:108838.
- [CrossRef] [PubMed] [Google Scholar]
- Large anterior temporal Virchow-Robin spaces: Unique MR imaging features. Neuroradiology. 2015;57:491-9.
- [CrossRef] [PubMed] [Google Scholar]





