Translate this page into:
The Yield of Repeat Angiography in Angiography-Negative Spontaneous Subarachnoid Hemorrhage
Prabhuraj A.R., MCh Department of Neurosurgery, National Institute of Mental Health and Neurosciences Bengaluru 560029, Karnataka India drprabhuraj@yahoo.co.in
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objective Despite the technological advancement in imaging, digital subtraction angiography (DSA) remains gold standard imaging modality for spontaneous subarachnoid hemorrhage (SAH). But even after DSA, around 15% of SAH remains elusive for the cause of the bleed. This is an institutional review to solve the mystery, “when is second DSA really indicated?”
Methods In a retrospective review from January 2015 to December 2017, we evaluated cases of spontaneous SAH with initial negative DSA with repeat DSA after 6 weeks to rule out vascular abnormality. The spontaneous SAH was confirmed on noncontrast computed tomography (NCCT) and divided into two groups of perimesencephalic SAH (PM-SAH) or nonperimesencephalic SAH (nPM-SAH). The outcome was assessed by a modified Rankin’s score (mRS) at 6 months postictus.
Results During the study period, we had 119 cases of initial negative DSA and 98 cases (82.3%) underwent repeat DSA after 6 weeks interval. A total of 53 cases (54.1%) had PM-SAH and 45 cases (45.9%) had nPM-SAH. Repeat DSA after 6 weeks showed no vascular abnormality in 53 cases of PM-SAH and in 2 (4.4%) out of 45 cases of nPM-SAH. At 6 months postictus, all cases of PM-SAH and 93% of nPM-SAH had mRS of 0.
Conclusion We recommend, a repeat DSA is definitely not required in PM-SAH, but it should be done for all cases of nPM-SAH, before labeling them as nonaneurysmal SAH. Although the overall outcome for nonaneurysmal spontaneous SAH is better than aneurysmal SAH, nPM-SAH has poorer eventual outcome compared to PM-SAH.
Keywords
angiography negative
SAH
repeat DSA
perimesencephalic SAH
outcomes
Introduction
The commonest cause of spontaneous subarachnoid hemorrhage (SAH) is intracranial aneurysm rupture. The most dreaded complication of a ruptured aneurysm is rebleed. Therefore, detecting an aneurysm in a patient with SAH is crucial. Although several modalities are used to detect aneurysm, digital subtraction angiography (DSA) is the gold standard.1 2 When a vascular abnormality is not detected, the diagnosis is labeled as angiography-negative (angionegative) SAH. Patients with angionegative SAH have superior survival and overall outcomes.3 Studies have shown that the initial angiogram is negative for the cause of SAH in 15 to 20% of the patients.4 5 Depending on the pattern of blood distribution, SAH can be divided into two categories: (1) perimesencephalic SAH (PM-SAH), which is restricted to PM or prepontine cistern; and (2) nonperimesencephalic SAH (nPM-SAH) which is a diffuse variety with bleeding extending into the anterior interhemispheric or Sylvian cisterns along with other basal cisterns. Compared with PM-SAH, nPM-SAH is having the higher possibility of aneurysm rupture.6 7 8 Currently, no consensus among either neurosurgeons or neuroradiologists exists on the need for further evaluation after the initial negative angiogram. Most institutes worldwide follow individualized policies based on personal experiences, which may vary from patient to patient. At our institute, a DSA is performed to detect aneurysm or any other vascular abnormality in patients with SAH. A second angiography is performed after 6 weeks of the first angiography in patients with angionegative SAH. We reviewed the results of the second angiography to determine its likelihood to detect an aneurysm.
Materials and Methods
This was a retrospective observational study of angionegative SAH cases, which were evaluated in a high-volume center for SAH treatment. In our institute, approximately 250 patients with aneurysm undergo surgical clipping and 50 patients undergo coiling annually. The study period was January 2015 to December 2017. Because this was a retrospective review of medical records, approval from the institute’s ethics committee was not required.
Literature Review
The electronic database MEDLINE (PubMed) was searched for relevant studies for the review. The search was performed using keywords such as “angio-negative subarachnoid hemorrhage,” “repeat angiography,” “digital subtraction angiography,” and “perimesencephalic subarachnoid hemorrhage.” All articles, published in English language from January 2000 to December 2017, were reviewed by a senior consultant neurosurgeon before inclusion.
Cerebral Angiography Technique
At our institute, the investigation of choice for SAH evaluation is DSA. A standard four-vessel DSA is performed on biplane angiography suite with three-dimensional (3D) rotational reconstruction. The femoral artery is punctured using the Seldinger technique, and 3D rotational angiography is performed with 21 mL of contrast injected at a rate of 3 mL/sec through a pressure injector with a maximum pressure of 250 psi without any delay. Nonionic iodinated contrast medium is used for the procedure, with the maximum amount determined according to the body weight of the patient asper ACR guidelines and adhering to the as low as reasonably achievable principle.
Evaluation Protocol
The reports of the patients who underwent DSA for the evaluation of spontaneous SAH were reviewed. From these reports, cases with angionegative SAH were identified. The clinical and imaging records of the patients with angionegative SAH of initial and repeat DSA at 6 weeks were reviewed independently by both the neuroradiologist and neurosurgeon. The modified World Federation of Neurosurgical Societies (WFNS) grade for clinical grading9 and modified Fisher’s grade10 for SAH severity on computed tomography (CT) scan were noted.
Definition of PM-SAH and nPM-SAH
The hemorrhage pattern of SAH was classified as per the criteria of Rinkel et al6 7 8 into following two groups: PM-SAH and nPM-SAH. In PM-SAH, the blood is seen immediately anterior to the midbrain, with or without extension of the blood to the anterior part of the ambient cistern or to the basal part of the Sylvian fissures, and no complete filling of the anterior interhemispheric fissure and extension to the lateral Sylvian fissures with no more than a minute amount of blood is observed. The sedimentation of a small amount of intraventricular blood in the occipital horns was allowed but not frank intraventricular hemorrhage.6 nPM-SAH was defined as SAH that did not meet all of the aforementioned criteria and had a more widespread distribution of the subarachnoid blood, resembling aneurysmal SAH.
Treatment Protocol
All patients underwent clinical and laboratory evaluation for the potential causes of intracranial bleed, including history of antiplatelet or anticoagulant medications, history of trauma, liver, and renal function tests, and coagulation profile (platelet count, prothrombin time, and activated partial thrombin time). All patients received medical management after admission. They were started on intravenous fluids, analgesics, and nimodipine tablet of 60 mg orally at 6 hourly for 21 days with adequate bed rest. Patients with persistent hydrocephalus on follow-up CT brain imaging were managed with either temporary cerebrospinal fluid (CSF) diversion or permanent diversion with external ventricular drain (EVD) or ventriculoperitoneal (VP) shunt, respectively. Following the second angiography if any vascular abnormality is noticed as per the pathology, it is treated with either microneurosurgery or endovascular means.
The outcome was assessed using the modified Rankin score (mRS) at 6 months after the ictus. The mRS was derived from the description of the clinical condition of the patient at follow-up, and the grading was assigned by the attending consultant neurosurgeon.
Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation, and categorical variables were expressed as counts and percentages. All analyses were performed using SPSS software (version 21.0, SPSS Inc.)
Results
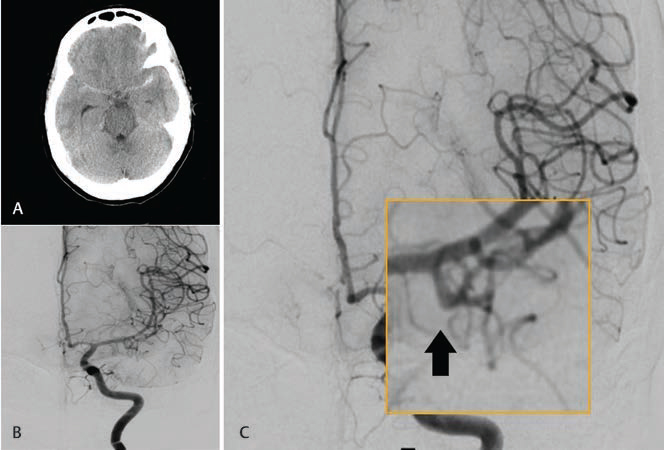
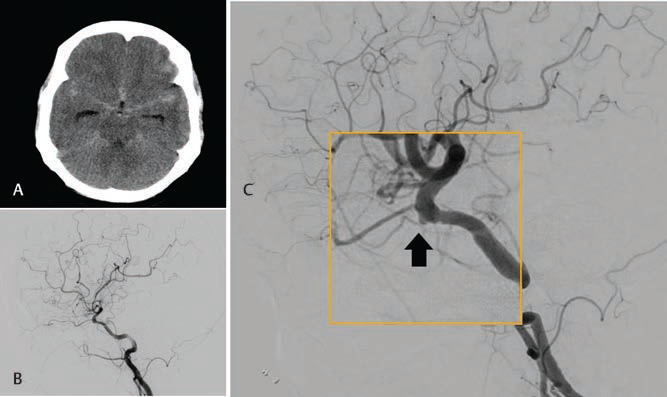
During the study period, 748 patients underwent DSA for the evaluation of SAH. Among them, 119 patients had angionegative SAH. Ninety-eight patients underwent repeat DSA at 6 weeks and were considered for analysis, while the remaining 21 were lost to follow-up. All patients had spontaneous SAH; none of them had a history of antiplatelet/anticoagulant use, trauma, or hepatic, renal, or neurological disease. The mean age of the cohort was 49.9 years (standard deviation [SD]: 48.8 ± 11.6), and 54.1% of the patients were male. No difference in age or gender was observed between the PM-SAH and nPM-SAH groups. Patients with nPM-SAH had a higher WFNS grade than those with PM-SAH; however, the Fisher grade was similar in both the groups (Table 1). After repeat angiography, 2 of 98 (2%) DSA patients showed an abnormality. Both the patients had nPM-SAH. One of the two patients was a 39-year-old female who had a headache for 3 days and persistent vomiting. At presentation, she had a modified WFNS grade 1 and modified Fisher’s grade 1 SAH (Fig. 1A). Initial DSA performed on the day of presentation was negative for any vascular abnormality (Fig. 1B). On second DSA, she was diagnosed with irregularity at the origin of the left anterior temporal artery (Fig. 1C). The other patient was a 58-year-old female who presented with thunder clapping headache with no comorbidities. She had modified WFNS grade 2 on presentation and modified Fisher’s grade 2 (Fig. 2A). Initial DSA performed on the day of presentation was negative for any vascular abnormality (Fig. 2B). On second DSA, she was diagnosed to have an irregularity at the origin of the ophthalmic segment of the internal carotid artery. This was an inferiorly directed bleb just distal to the distal dural ring. (Fig. 2C). As the irregularities were nonsignificant and not suggestive of a definite aneurysm, no specific intervention was offered. The outcome of angionegative SAH was positive. Only three (3%) patients had an mRS grade-1 outcome, whereas the rest had grade 0 outcome. All these three cases had nPM-SAH. Hydrocephalus was observed in seven patients (7.14%) in our cohort, which was managed with temporary CSF diversion with EVD. Two of them needed permanent CSF diversion; they underwent VP shunt placement (Fig 3).
|
PM-SAH n (%) |
nPM-SAH n (%) |
p-Value |
|
|---|---|---|---|
|
Abbreviations: DSA, digital subtraction angiography; F, female; M, male; mWFNS, modified World Federation of Neurosurgical Society grade; mRS, modified Rankin’s scale; nPM-SAH, nonperimesencephalic subarachnoid hemorrhage; SD, standard deviation. |
|||
|
n = 98 |
53 (54.1%) |
45 (45.9%) |
0.398 |
|
M:F = 53:45 |
28:25 |
25:20 |
0.934 |
|
Age in year (mean ± SD) 49.9 ±12.2 |
48.8 ± 11.6 |
51.2 ± 12.9 |
– |
|
False-negative DSA 2 (2%) |
0 |
2 (4.4%) |
0.167 |
|
mWFNS grade n (%) |
0.408 |
||
|
I 43 (43.8) |
28 (52.8) |
15 (33.3) |
|
|
II 28 (28.5) |
19 (35.8) |
9 (20) |
|
|
III 23 (23.5) |
5 (9.4) |
18 (40) |
|
|
IV 4 (4.1) |
1 (1) |
3 (6.6) |
|
|
V 0 (0) |
0 |
0 |
|
|
Modified Fischer’s grade n (%) |
0.786 |
||
|
I 37 (37.7) |
20 (37.7) |
17 (37.7) |
|
|
II 23 (23.4) |
10 (18.8) |
13 (28.8) |
|
|
III 21 (21.4) |
12 (22.6) |
9 (20) |
|
|
IV 23 (17.3) |
17 (20.7) |
6 (13.3) |
|
|
mRS at 6 months |
0.112 |
||
|
0 |
53 (100) |
42 (93.3) |
|
|
1 |
0 |
3 (6.6) |
|
-
Fig. 1 A 39-year-old woman presented with headache and vomiting. (A) NCCT brain axial image showing predominantly left Sylvian fissure SAH. (B) DSA image on the anteroposterior projection of left ICA injection showing normal filling of both ACA and MCA without any obvious vascular abnormality. (C). Magnified image of repeat DSA at 6 weeks on the anteroposterior projection of left ICA injection showing normal filling of ACA and MCA. A bleb is seen on the anterior temporal artery (arrow). ACA, anterior cerebral artery; DSA, digital subtraction angiography; ICA, internal cerebral artery; MCA, middle cerebral artery; NCCT, noncontrast computed tomography; SAH, subarachnoid hemorrhage.
Fig. 1 A 39-year-old woman presented with headache and vomiting. (A) NCCT brain axial image showing predominantly left Sylvian fissure SAH. (B) DSA image on the anteroposterior projection of left ICA injection showing normal filling of both ACA and MCA without any obvious vascular abnormality. (C). Magnified image of repeat DSA at 6 weeks on the anteroposterior projection of left ICA injection showing normal filling of ACA and MCA. A bleb is seen on the anterior temporal artery (arrow). ACA, anterior cerebral artery; DSA, digital subtraction angiography; ICA, internal cerebral artery; MCA, middle cerebral artery; NCCT, noncontrast computed tomography; SAH, subarachnoid hemorrhage.
-
Fig. 2 A 58-year-old woman presented with thunder clapping headache. (A) NCCT brain axial image showing diffuse SAH involving Anterior Inter-hemispheric and bilateral Sylvian SAH. (B) DSA image on a lateral projection of left ICA injection showing normal filling of both ACA and MCA without any obvious vascular abnormality.(C) Magnified image of repeat DSA at 6 weeks on a lateral projection of left ICA injection showing a bleb on the ICA just proximal to the origin of the ophthalmic artery (arrow). ACA, anterior cerebral artery; DSA, digital subtraction angiography; ICA, internal cerebral artery; MCA, middle cerebral artery; NCCT, noncontrast computed tomography; SAH, subarachnoid hemorrhage.
Fig. 2 A 58-year-old woman presented with thunder clapping headache. (A) NCCT brain axial image showing diffuse SAH involving Anterior Inter-hemispheric and bilateral Sylvian SAH. (B) DSA image on a lateral projection of left ICA injection showing normal filling of both ACA and MCA without any obvious vascular abnormality.(C) Magnified image of repeat DSA at 6 weeks on a lateral projection of left ICA injection showing a bleb on the ICA just proximal to the origin of the ophthalmic artery (arrow). ACA, anterior cerebral artery; DSA, digital subtraction angiography; ICA, internal cerebral artery; MCA, middle cerebral artery; NCCT, noncontrast computed tomography; SAH, subarachnoid hemorrhage.
-
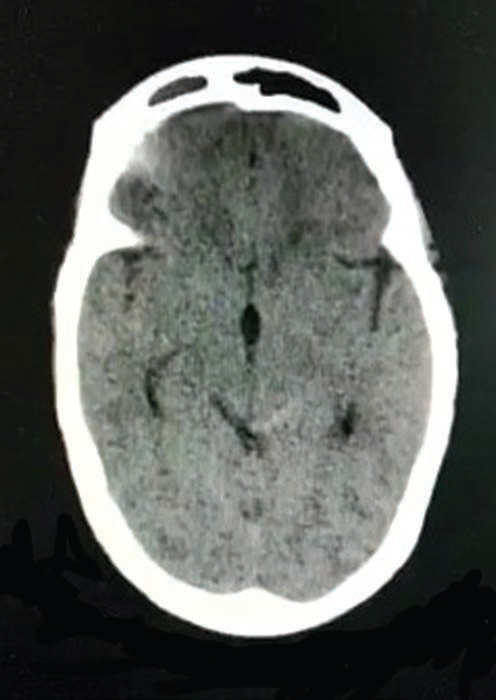
Fig. 3 A 42-year-old man presented with once in a lifetime severe headache. NCCT axial brain image showing left-sided perimesencephalic SAH. NCCT, noncontrast computed tomography; SAH, subarachnoid hemorrhage.
Fig. 3 A 42-year-old man presented with once in a lifetime severe headache. NCCT axial brain image showing left-sided perimesencephalic SAH. NCCT, noncontrast computed tomography; SAH, subarachnoid hemorrhage.
Discussion
The clinical presentation of spontaneous SAH is similar irrespective of etiology. However, the clinical course and outcome are significantly worse in aneurysmal SAH. The clinical outcome in angionegative SAH is excellent with a low risk of vasospasm and hydrocephalus.3 Therefore, identifying the cause of SAH is crucial. An undetected aneurysmal SAH may be associated with rebleeding risk, resulting in mortality and morbidity. PM-SAH is a well-known entity associated with superior outcomes. In most cases, PM-SAH is not caused by rupture of an aneurysm.
PM-SAH was first described by van Gijn et al11 in 1985 as a distinct entity, negative DSA, and a benign clinical course with less risk of rebleeding and vasospasm.12 13 Rinkel et al attributed this pattern of SAH to a ruptured posterior circulation aneurysm in up to 5% patients,6 although rates as low as 1.3% have been reported.14 According to the series by Kallmes et al in 1996, ruptured posterior circulation aneurysm can present with PM-SAH in up to 12.4% patients.15 The etiology of nonaneurysmal SAH remains unknown, but it is presumed to have a venous origin in most cases like a tear in the basal vein of Rosenthal or one of its tributaries, anterior longitudinal pontine, or interpeduncular and posterior communicating veins.16 Various other theories for the source of bleeding includes a capillary leak, lenticulostriate, or thalamoperforating arteries, intraluminal basilar dissection, cryptic brainstem arterio venous malformations (AVMs), and capillary telangiectasia and cavernous malformations.17 18 Similar to the findings in our study, majority of the studies have reported the rate of false-negative angiography among patients with PM-SAH to be 0%. In patients with nPM-SAH, the source of bleed can be occult aneurysm, vascular malformation, intracranial arterial dissection, and cerebral venous thrombosis. Considering the lack of sufficient evidence on nPM-SAH management and the associated high risk of vasospasm and hydrocephalus, performing a repeat evaluation for finding the cause of SAH is crucial.19 Overall, the most common cause of missed vascular pathology detected on repeat angiography is anterior communicating artery (ACoA) aneurysms.20 In our study, none of the patients with positive PM-SAH on the second angiography had vascular abnormality related to any specific vascular territory or ACoA region as mentioned in the literature. The angiograms can yield false-negative results for various reasons, such as vasospasm, vascular thrombosis, obliteration of the aneurysm due to the effects of an adjacent hematoma, or technically inadequate examinations.1 21 Therefore, even when the results of the first angiography are negative, a second DSA is mandatory before diagnosing the patients to have nonaneurysmal nPM-SAH. Patients with nonaneurysmal nPM-SAH have a superior prognosis compared with those with aneurysmal SAH but not superior to those with PM-SAH.4 Currently, no internationally accepted guidelines exist for repeat angiography for initial angionegative SAH. In most institutions, repeat evaluation is performed at the discretion of the treating physician. Multiple studies have assessed the utility of repeat angiography; however, most of them have advised that repeat evaluation in the PM-SAH group is only required in patients with significant vasospasm or inadequate initial DSA. By contrast, in patients with nPM-SAH, repeat angiography is performed for almost every case considering the high probability of finding a potential underlying treatable vascular pathology.22 23 At our institute, we have a standardized protocol for DSA that includes anteroposterior and lateral views with rotational images and 3D reconstruction of all vessels injected. Moreover, adequate opacification of ACoA and posterior inferior cerebellar artery origin is achieved in all evaluations. The images are reviewed independently by neuroradiologists and neurosurgeons before reporting them as angionegative SAH. This reduces the chances of false-negative evaluation.
In the present study, we found initial false-negative angiogram in 4.4 and 0% of the nPM-SAH and PM-SAH groups, with a false-negative rate of 2% for the cohort. As per the literature, the detection rate of an underlying cause of SAH with repeat angiogram ranges from 0 to 17%.23 24 25 Table 2 shows the yield of repeat DSA of some of the recent studies.
|
Study (year) |
Type of study |
Study time frame |
n |
Diagnostic yield (%) |
Timing of repeat DSA |
Outcome assessment at follow-up (mo) |
|---|---|---|---|---|---|---|
|
Abbreviations: DSA, digital subtraction angiography; nPM-SAH, nonperimesencephalic subarachnoid hemorrhage; NR, not reported; Pros, prospective; Retro, retrospective. Note: two values represent the number of patients at the first and second repeat DSA. |
||||||
|
Topcuoglu et al (2003)1 |
Retro |
1995–2002 |
41 |
4 (9.75) |
12 d and 5 wk |
NR |
|
Little et al (2007)26 |
Pros |
2003–2006 |
42 |
4/1 (12) |
7 d and 6 wk |
NR |
|
Andaluz and Zuccarello (2008)2 |
Retro |
1998–2003 |
47 |
7 (14.9) |
NR |
NR |
|
Gupta et al (2009)27 |
Pros |
2005–2006 |
34 |
2 (5.88) |
6 wk |
20 |
|
Prestigiacomo et al (2010)28 |
Pros |
2002–2005 |
13 |
0 (0) |
NR |
NR |
|
Agid et al (2010)29 |
Retro |
2005–2009 |
28 |
4 (14) |
NR |
NR |
|
Almandoz et al (2012)13 |
Pros |
2005–2010 |
39 |
2 (5.1) |
7 d |
NR |
|
Yu et al (2012)30 |
Retro |
2005–2012 |
12 |
2(16.7) |
NR |
NR |
|
Dalyai et al (2013)24 |
Retro |
2003–2011 |
136 |
10/7 (12.5) |
7 d and 6 wk |
NR |
|
Moscovici et al (2013)14 |
Retro |
2005–2011 |
31 |
2 (6.45) |
7–10d |
6 |
|
Khan et al (2013)31 |
Retro |
2008–2012 |
6 |
0 (0) |
23 d |
6 |
|
Bakker et al (2014)25 |
Retro |
2005–2012 |
45 |
0 |
14 d |
NR |
|
Kumar (2014)23 |
Pros |
2011–2013 |
17 |
2 (11.76) |
6 wk |
6 |
|
Bashir et al (2018)22 |
Retro |
2011–2017 |
35 |
1 (2.86) |
7–23 d |
3 |
|
Present Study |
Retro |
2015–2017 |
45 |
2 (4.4) |
6 wk |
6 |
Despite the study findings, the timing of the repeat evaluation and the type of angiography have been debated. No randomized study has analyzed DSA versus CT angiography (CTA) versus magnetic resonance angiogram (MRA) directly in an initial negative evaluation. Topcuoglu et al, in their study in 2003, investigated the diagnostic yield of repeat DSA and other neuroimaging studies in 86 patients with angionegative SAH and detected the source of bleeding in four patients with nPM-SAH through repeat DSA. They concluded that DSA was the only subsequent imaging that could reveal the cause of hemorrhage, particularly in patients with nPM-SAH.1 Another study by Delgado Almandoz et al in 2012 evaluated the yield of CTA or MRA in initial negative DSA. They detected only one aneurysm in 73 MRA cases (1.36% yield) and four aneurysms in 77 CTA cases (5.2% yield).13 The CTA might be a useful adjunct procedure for subsequent investigations following a negative initial DSA, with a reported pooled sensitivity and specificity up to 97%.32 The CTA has some advantages over DSA in terms of invasiveness, availability, radiation exposure, and examination time. With advances in the CTA technology, it is favored over DSA by many not just for a repeat but even for primary evaluation. Additionally, DSA is an invasive procedure that carries a 0.5 to 1.8% risk of neurological complications with a permanent deficit in 0.09 to 0.5%.20 25 In addition, nonneurological complications, including groin hematoma, peripheral thromboembolism, transient hypotension, and arteriovenous fistula, occur in 0.6% of the patients.25 DSA is the gold standard1 2 and should be performed in case of any uncertainty pertaining to CTA, particularly for small aneurysms <4 mm in diameter. Posterior circulation aneurysms as artifacts due to bony anatomy can lead to misdiagnosis in CTA.27 33 With advancement in CTA techniques and according to a meta-analysis, CTA has shown a pooled sensitivity and specificity of 98 and 100%, respectively, for 16- or 64-detector CTA.34 With this high sensitivity of CTA, if the initial DSA is of superior quality with no evidence of severe hemorrhage, vasospasm, and thrombosis concealing the aneurysm or AVM, a repeat DSA may not be required. In such cases, CTA may be a superior choice for patients with angionegative SAH for initial or follow-up evaluation.29 Compared with CTA, most studies using MRA as the mode of angiography for evaluation following initial negative angiography have shown a yield of 0%.1 2 Overall, the outcome has been reasonable for nonaneurysmal SAH compared with aneurysmal SAH. The PM-SAH group presented superior outcomes compared with the nPM-SAH group. In our cohort, all 53 patients were completely asymptomatic at 6 months of follow-up in the PM-SAH group (mRS grade 0), and 93.3% of the patients in the nPM-SAH group were asymptomatic (mRS grade 0). Only three patients (6.67%) in the nPM-SAH group had a minor disability at 6 months. Studies with their outcomes are mentioned in Table 3.
|
Study |
Outcome assessment |
PM-SAH (%) |
nPM-SAH (%) |
|---|---|---|---|
|
Abbreviations: GOS, Glasgow outcome scale; mRS, modified Rankin scale; nPM-SAH, non-perimesencephalic subarachnoid hemorrhage. aAt discharge. |
|||
|
Gupta et al (2009)27 |
GOS |
100 |
94.1 |
|
Moscovici et al (2013)14 |
mRS |
100 |
68 |
|
Khan et al (2013)31 |
GOS |
100 |
86.9 |
|
Kumar et. al (2014)23 |
mRS |
95 |
83.3 |
|
Bashir et al (2017)22 a |
mRS |
100 |
89 |
|
Present study |
mRS |
100 |
93.3 |
Limitations
The main limitations of our study were the retrospective design and less number of patients. A prospective study with a relatively large number of patients with angionegative SAH is warranted to examine the course of the disease and determine the optimal timing of repeat DSA. The second limitation was the timing of repeat DSA. The cumulative incidence of rebleeds from an undiagnosed ruptured aneurysmal SAH was significantly high after 6 weeks, and a repeat angiogram was therefore performed much sooner in several centers, particularly in nPM-SAH-type bleeds. The time required may be 1 or 2 days and upwards, depending on the clinical appearance and standard operating procedures. We did not perform any CTA or MRA between the first and follow-up DSAs for any patient. Additionally, the reported 6 weeks may be considered too long to rule out small blister aneurysms, a known severe type of aneurysm that is difficult to diagnose and treat, which is also prone to rebleeds. However, no cases of rebleeds were noted in the initial negative DSA patients in any group. Another limitation was lack of follow-up of an entire cohort; almost one-fifth of the initial cases were lost to follow-up. The status of repeat DSA, as well as clinical condition could not be determined in these cases.
Conclusion
As 100% of the patients with PM-SAH showed negative results on repeat angiogram, repeat evaluation is not necessary, although for confirmation follow-up evaluation with CTA/MRA can be performed. Patients with nPM-SAH presented a 4.4% risk of initial false-negative angiography; therefore, repeat DSA should be performed for all. The timing of the repeat evaluation is debatable, but we prefer repeat evaluation at 6 weeks after the initial bleed.
Note
Part of the data was presented in electronic-poster format at NSICON 2017, Nagpur, India.
Conflict of Interest
None declared.
Funding None.
References
- Subarachnoid hemorrhage without evident cause on initial angiography studies: diagnostic yield of subsequent angiography and other neuroimaging tests. J Neurosurg. 2003;98(6):1235-1240.
- [Google Scholar]
- Yield of further diagnostic work-up of cryptogenic subarachnoid hemorrhage based on bleeding patterns on computed tomographic scans. Neurosurgery. 2008;62(5):1040-1046, discussion 1047.
- [Google Scholar]
- Non-aneurysmal spontaneous subarachnoid hemorrhage: perimesencephalic versus non-perimesencephalic. (in Portuguese) Rev Bras Ter Intensiva. 2016;28(2):141-146.
- [Google Scholar]
- Comparison between perimesencephalic nonaneurysmal subarachnoid hemorrhage and subarachnoid hemorrhage caused by posterior circulation aneurysms. J Neurosurg. 2003;98(3):529-535.
- [Google Scholar]
- Ruptured cerebral aneurysms missed by initial angiographic study. Neurosurgery. 1990;27(1):45-51.
- [Google Scholar]
- Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol. 1991;12(5):829-834.
- [Google Scholar]
- Hasan D, Brouwers PJAM, van Gijn J. The clinical course of perimesencephalic nonaneurysmal subarachnoid hemorrhage. Ann Neurol. 1991;29(5):463-468.
- [Google Scholar]
- Subarachnoid hemorrhage without detectable aneurysm. A review of the causes. Stroke. 1993;24(9):1403-1409.
- [Google Scholar]
- Modified World Federation of Neurosurgical Societies subarachnoid hemorrhage grading system. World Neurosurg. 2015;83(5):801-807.
- [Google Scholar]
- Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59(1):21-27, discussion 21–27.
- [Google Scholar]
- Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985;35(4):493-497.
- [CrossRef] [Google Scholar]
- Hageman LM, Tans JT, van Gijn J. Outcome in perimesencephalic (nonaneurysmal) subarachnoid hemorrhage: a follow-up study in 37 patients. Neurology. 1990;40(7):1130-1132.
- [Google Scholar]
- Diagnostic yield of computed tomography angiography and magnetic resonance angiography in patients with catheter angiography-negative subarachnoid hemorrhage: clinical article. J Neurosurg. 2012;117(2):309-315.
- [Google Scholar]
- Clinical relevance of negative initial angiogram in spontaneous subarachnoid hemorrhage. Neurol Res. 2013;35(2):117-122.
- [Google Scholar]
- Ruptured vertebrobasilar aneurysms: frequency of the nonaneurysmal perimesencephalic pattern of hemorrhage on CT scans. Radiology. 1996;201(3):657-660.
- [Google Scholar]
- Concurrent presentation of perimesencephalic subarachnoid hemorrhage and ischemic stroke. J Stroke Cerebrovasc Dis. 2008;17(4):248-250.
- [Google Scholar]
- Perimesencephalic nonaneurysmal subarachnoid hemorrhage caused by jugular venous occlusion: case report. Neurosurgery. 2008;63(6):E1202-E1203, discussion E1203.
- [Google Scholar]
- Perimesencephalic nonaneurysmal hemorrhage associated with vein of Galen stenosis. Neurology. 2008;70:2410-2411. (24 Pt 2, 24 Pt 2)
- [Google Scholar]
- Origin of pretruncal nonaneurysmal subarachnoid hemorrhage: ruptured vein, perforating artery, or intramural hematoma? Mayo Clin Proc. 2000;75(11):1169-1173.
- [CrossRef] [Google Scholar]
- Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227(2):522-528.
- [Google Scholar]
- von Weymarn A, Radü EW, Steinbrich W. Value of repeat-angiography in cases of unexplained subarachnoid hemorrhage (SAH) Acta Neurol Scand. 1996;93(5):366-373.
- [Google Scholar]
- Non-aneurysmal subarachnoid hemorrhage: When is a second angiography indicated? Neuroradiol J. 2018;31(3):244-252.
- [Google Scholar]
- Angio negative spontaneous subarachnoid hemorrhage: Is repeat angiogram required in all cases? Surg Neurol Int. 2014;5(1):125.
- [Google Scholar]
- Subarachnoid hemorrhage with negative initial catheter angiography: a review of 254 cases evaluating patient clinical outcome and efficacy of short- and long-term repeat angiography. Neurosurgery. 2013;72(4):646-652, discussion 651–652.
- [Google Scholar]
- Repeat digital subtraction angiography after a negative baseline assessment in nonperimesencephalic subarachnoid hemorrhage: a pooled data meta-analysis. J Neurosurg. 2014;120(1):99-103.
- [Google Scholar]
- Evaluation of patients with spontaneous subarachnoid hemorrhage and negative angiography. Neurosurgery. 2007;61(6):1139-1150, discussion 1150–1151.
- [Google Scholar]
- Nonaneurysmal nonperimesencephalic subarachnoid hemorrhage: is it a benign entity? Surg Neurol. 2009;71(5):566-571, discussion 571, 571–572, 572.
- [Google Scholar]
- Three dimensional CT angiography versus digital subtraction angiography in the detection of intracranial aneurysms in subarachnoid hemorrhage. J Neurointerv Surg. 2010;2(4):385-389.
- [Google Scholar]
- Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: When is digital subtraction angiography still needed? AJNR Am J Neuroradiol. 2010;31(4):696-705.
- [Google Scholar]
- Subarachnoid hemorrhage with negative baseline digital subtraction angiography: is repeat digital subtraction angiography necessary? J Cerebrovasc Endovasc Neurosurg. 2012;14(3):210-215.
- [Google Scholar]
- Angiogram negative subarachnoid haemorrhage: outcomes and the role of repeat angiography. Clin Neurol Neurosurg. 2013;115(8):1470-1475.
- [Google Scholar]
- Velthuis BK, van Gijn J. Perimesencephalic hemorrhage and CT angiography: A decision analysis. Stroke. 2000;31(12):2976-2983.
- [Google Scholar]
- Detection of aneurysms by 64-section multidetector CT angiography in patients acutely suspected of having an intracranial aneurysm and comparison with digital subtraction and 3D rotational angiography. AJNR Am J Neuroradiol. 2008;29(3):594-602.
- [Google Scholar]
- Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis–systematic review and meta-analysis. Radiology. 2011;258(1):134-145.
- [Google Scholar]






