Translate this page into:
The Non-motor Symptoms, Disability Progression, and Survival Analysis of Atypical Parkinsonism: Case Series from Eastern India and Brief Review of Literature
Tapas Pani, MD, DM Department of Neurology, SCB Medical College and Hospital Cuttack 753101, Odisha India tapaspani625@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objective The objectives of this study are (1) to describe the non-motor profile, the motor disability progression, and survival analysis of atypical parkinsonism in a tertiary care hospital of eastern India and (2) to elucidate the neurocircuitry and the putative substrates responsible for non-motor manifestations.
Methods In this prospective observational study, patients were diagnosed based on Consensus Criteria for Progressive Supranuclear Palsy (PSP), The Fourth Consensus Report of the Dementia with Lewy Body (DLBD) Consortium 2017, The Autonomic Neuroscience 2018 Criteria for Multiple System Atrophy (MSA), and Armstrong 2013 Criteria for Corticobasal Degeneration (CBD). Disease severity was assessed at baseline and 6 months of follow-up using the Unified Parkinson's Disease Rating Scales (UPDRS). For PSP and MSA, the PSP-Clinical Deficits Scale (PSP-CDS) and the Unified MSA Rating Scale (UMSARS), respectively, were used. Cox regression analysis and the hazard ratio were calculated.
Results Out of 27 patients, the diagnosis was probable PSP in 12, probable MSA in 7, probable CBD in 5, and probable DLBD in 3. Non-motor symptoms were highly prevalent across all subtypes. Motor disability progression as assessed by UPDRS parts 2 and 3 showed significant deterioration over 6-month follow-up across all groups (p < 0.05). Disease progression assessed by PSP-CDS and UMSARS over 6 months was significant (p < 0.05). One PSP and two MSA patients died during a 6-month follow-up period. The hazard ratio in MSA was 3.5 (95% confidence interval: 0.31–0.38) with p = 0.306.
Conclusion Atypical parkinsonian disorders are rare, and usually more severe than idiopathic parkinsonism. As no definitive treatment is available, symptomatic management involving a multidisciplinary team approach must be prioritized.
Keywords
atypical parkinsonism
progressive supranuclear palsy
survival
non-motor symptoms
multiple system atrophy
corticobasal degeneration
Introduction
Atypical parkinsonism encompasses progressive supranuclear palsy (PSP), multiple system atrophy (MSA), dementia with Lewy body (DLBD), and corticobasal degeneration (CBD) and is characterized by rapid disease progression, poor levodopa responsiveness, shorter survival time, and more complications in earlier stages and with a higher degree of severity than in idiopathic Parkinson's disease (IPD).1 The non-motor symptoms (NMS) are extremely common in atypical parkinsonism; however, these are underappreciated and undertreated. The underlying mechanism involves the involvement of multiple areas of neuraxis from the central nervous system to the peripheral nervous system.1
Distinct neural representations of depression, anxiety, apathy, and fatigue have been elucidated.2 The disruption of the noradrenergic projections from the locus coeruleus is implicated in the pathogenesis of depression, anxiety, apathy, decreased memory consolidation and retrieval, and poor rapid eye movement (REM) sleep.3 Apathy stems from the involvement of the mesocortical, mesolimbic, and nigrostriatal pathways. Cortical areas implicated are the orbitofrontal cortex, subgenual portions of the anterior cingulate cortex, and dorsolateral and ventrolateral prefrontal cortex along with caudate, putamen, and globus pallidus.4
According to the Chaudhuri and Behan model of basal ganglia dysfunction in central fatigue, dorsal striatal areas and cortical–subcortical networks contribute to perceptions of fatigue due to disruptions of internally generated effort.5
MSA has the highest prevalence of pain; characterization of pain was mainly musculoskeletal throughout all subtypes. In CBD, dystonic pain along with central pain was most common; while in DLBD, multilocalized pain is highly prevalent.6 Neurodegeneration affecting the basal ganglia alters pain perception as it participates in pain processing, hence the higher prevalence in MSA-parkinsonism (MSA-P) versus MSA-cerebellar (MSA-C). Cognitive impairment in PSP may reduce pain perception.
Symptomatic orthostatic hypotension, the major manifestation of cardiovascular autonomic failure, often manifests as recurrent syncope, dizziness, nausea, headache, and weakness, and has been reported in 43 to 81% of all MSA patients. Three main mechanisms include noradrenergic denervation in the cardiac and extracardiac regions and arterial baroreflex failure7 8 (Fig. 1).
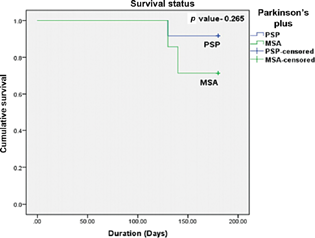
-
Fig. 1 Kaplan-Meier survival curve. MSA, multiple system atrophy; PSP, progressive supranuclear palsy.
Fig. 1 Kaplan-Meier survival curve. MSA, multiple system atrophy; PSP, progressive supranuclear palsy.
Sleep disorders in the form of insomnia, REM sleep behavior disorder, periodic limb movement disorder, excessive daytime sleepiness, and sleep apneas are common in atypical parkinsonism.9 The putative substrates responsible for sleep disturbances are shown in Fig. 2 in the sleep–wake neurocircuitry.10

-
Fig. 2 Putative substrates underlying sleep disturbances in atypical parkinsonism. ABN, abnormal; BF, basal forebrain; DEC, decreased; DMH, dorsomedial hypothalamic nucleus; EDS, excessive daytime sleepiness; MAs, monoaminergic systems; MCH, melanin-concentrating hormone; PB/PC, parabrachial/preceruleus; PLH/vPAG, posterolateral hypothalamus/ventral periaqueductal area; RBD, rapid eye movement sleep behavior disorder; RLS, restless legs syndrome; SCN, suprachiasmatic nucleus; SII, spinal inhibitory interneuron; SLD, sublaterodorsal nucleus; vlPAG/LPT, ventrolateral periaqueductal/lateral pontine tegmentum; VLPO, ventrolateral preoptic area; Vm, ventral medulla. Note: Excitatory projections in solid lines, inhibitory ones in dashed lines.
Fig. 2 Putative substrates underlying sleep disturbances in atypical parkinsonism. ABN, abnormal; BF, basal forebrain; DEC, decreased; DMH, dorsomedial hypothalamic nucleus; EDS, excessive daytime sleepiness; MAs, monoaminergic systems; MCH, melanin-concentrating hormone; PB/PC, parabrachial/preceruleus; PLH/vPAG, posterolateral hypothalamus/ventral periaqueductal area; RBD, rapid eye movement sleep behavior disorder; RLS, restless legs syndrome; SCN, suprachiasmatic nucleus; SII, spinal inhibitory interneuron; SLD, sublaterodorsal nucleus; vlPAG/LPT, ventrolateral periaqueductal/lateral pontine tegmentum; VLPO, ventrolateral preoptic area; Vm, ventral medulla. Note: Excitatory projections in solid lines, inhibitory ones in dashed lines.
Three characteristic features define bladder abnormalities in MSA. These include large postvoid residual urine volumes of > 100 mL, an open bladder neck during filling-phase video urodynamics, and sphincter denervation attributed to neuronal cell loss in Onuf's nucleus in the sacral spinal segment.7 Urinary dysfunction in PSP is as extensive as those of MSA.11
The reduction of motor performance seems to contribute to the development of severe constipation.12 Therefore, the improvement of gait capacity and endurance could help reduce the risk of constipation.
Progression of motor disability is more rapid in atypical parkinsonism compared with IPD.1
In patients with atypical parkinsonism, the median survival was 3.3 years, compared with 5.6 years in controls.13
Materials and Methods
A prospective study including PSP, MSA, CBD, and DLBD patients was carried out. Patients were followed-up for 6 months, to assess their mortality.
The study was approved by the Institutional Ethical Committee and proceeded with the approval of the participant's consent.
Patients were diagnosed based on the Consensus Criteria for PSP (Movement Disorders Society 2017),14 the Fourth Consensus Report of the DLBD Consortium 2017,15 the MSA Diagnostic Criteria (Autonomic Neuroscience 2018),16 and the Armstrong Criteria for CBD.17 Disease severity was assessed at presentation and 6 months of the follow-up period. The data were analyzed using Statistical Package for the Social Sciences, version 23 (IBM Corp, Armonk, New York). Descriptive analysis was done for baseline characteristics of study patients. The pretest and posttest values of the Unified Parkinson Disease Rating Scale (UPDRS) parts 2 and 3, the PSP-Clinical Deficits Scale (PSP-CDS),18 and the Unified MSA Rating Scale (UMSARS)19 were compared and analyzed using paired t-test. The detailed clinical evaluation and the scoring were done by both the authors.
Survival analysis was done using Kaplan–Meier survival curve where the log-rank test was performed. Cox regression analysis was performed to get the hazard ratio. p-Value less than 0.05 was considered statistically significant.
Results
Mean age was higher in DLBD and CBD patients (69 ± 5.8 and 67 ± 1.7 years, respectively) compared with MSA and PSP (61 ± 6.7 and 65 ± 3.3 years, respectively) patients. The duration of the disease was similar across subgroups. The male to female ratio was 2.7:1. Among PSP patients, PSP-Richardson (PSP-RS) was the most common type (58.33%; Table 1).
|
Diseasea (no. of patients/deaths) |
Age at presentation |
Duration of disease |
Subtypes (%) |
M/F |
|---|---|---|---|---|
|
PSP (12/1) |
65 ± 3.3 |
3.7 ± 1.4 |
PSP-RS (58.33) PSP-PI (8.33) PSP-P (8.33) PSP-OM (16.67) PSP-F (8.33) |
9/3 |
|
MSA (7/2) |
61 ± 6.7 |
3.7 ± 0.8 |
MSA-P (28.57) MSA-C (71.42) |
5/2 |
|
DLBD (3/0) |
69 ± 5.8 |
3.7 ± 1.4 |
2/1 |
|
|
CBD (5/0) |
67 ± 1.7 |
2.6 ± 0.3 |
3/2 |
Abbreviations: CBD, corticobasal degeneration; DLBD, dementia with Lewy body; M/F. male/female; MSA, multiple system atrophy; MSA-C, MSA cerebellar; MSA-P, MSA parkinsonism; PSP, progressive supranuclear palsy; PSP-F, PSP frontal; PSP-OM, PSP oculomotor; PSP-P, PSP Parkinson's type; PSP-PI, PSP postural instability; PSP-RS, PSP Richardson.
MSA patients showed moderate to severe involvement in these NMS domains: depression (57.1%), apathy (57.1%), sleep disturbances (57.1%), bladder problems (71.4%), constipation (71.4%), lightheadedness (57.1%), and fatigue (57.1%). In PSP, cognitive disturbances (66.6%), apathy (75%), sleep disturbances (75%), bladder problems (58.3%), constipation (75%), and fatigue (66.7%) were highly prevalent. CBD patients were mildly affected across all NMS domains, except constipation (40%). The majority of DLBD patients showed marked to severe involvement across all NMS domains except bladder problems, lightheadedness, fatigue, and excessive daytime sleepiness (Table 2).
|
Domains (slight to mild/moderate to severe) UPDRS-scale-based scoring in % |
PSP |
MSA |
CBD |
DLBD |
|---|---|---|---|---|
|
Cognitive |
58.8/8.3 |
42.9/28.6 |
40/40 |
0/100 |
|
Hallucinations |
41.7/0 |
71.4/14.3 |
40/0 |
0/100 |
|
Depression |
33.3/25 |
28.6/57.1 |
40/20 |
0/66.7 |
|
Anxiety |
50/0 |
57.1/42.9 |
40/0 |
33.3/66.7 |
|
Apathy |
33.3/41.7 |
28.6/57.1 |
40/20 |
0/100 |
|
Sleep disturbances |
58.3/16.7 |
14.3/57.1 |
20/0 |
33.3/66.7 |
|
Excessive daytime sleepiness |
50/0 |
71.4/0 |
20/0 |
66.7/33.3 |
|
Pain |
41.7/0 |
42.9/28.6 |
40/0 |
33.3/66.7 |
|
Bladder |
58.3/0 |
0/71.4 |
20/20 |
66.7/0 |
|
Constipation |
50/25 |
14.3/71.4 |
20/40 |
33.3/66.7 |
|
Lightheadedness |
16.7/0 |
14.3/57.1 |
40/20 |
33.3/0 |
|
Fatigue |
50/16.7 |
28.6/57.1 |
20/0 |
66.7/0 |
Abbreviations: CBD, corticobasal degeneration; DLBD, dementia with Lewy body; MSA, multiple system atrophy; PSP, progressive supranuclear palsy; UPDRS, Unified Parkinson's Disease Rating Scale.
Disease progression as assessed by UPDRS parts 2 and 3, which showed a rapid deterioration in motor performance over a short period compared with typical Parkinson's patients (Table 3).
|
UPDRS 2 |
UPDRS 3 |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Initial |
Final |
Change |
p-Value |
Initial |
Final |
Change |
p-Value |
|
|
PSP |
20.27 ± 3.98 |
25.45 ± 3.83 |
5.18 ± 1.07 |
0.001 |
38.18 ± 3.66 |
44.82 ± 12.38 |
6.63 ± 3.04 |
0.001 |
|
MSA |
23 ± 8.69 |
29.8 ± 10.61 |
5 ± 1 |
0.001 |
29.4 ± 8.11 |
39 ± 4.64 |
6.4 ± 0.54 |
0.001 |
|
CBD |
20 ± 3.94 |
27.4 ± 4.62 |
7.4 ± 0.89 |
0.001 |
37.8 ± 10.87 |
45.8 ± 12.62 |
8 ± 2.73 |
0.003 |
|
DLBD |
22.67 ± 3.79 |
27 ± 3.46 |
4.33 ± 0.89 |
0.001 |
40.67 ± 9.07 |
48.33 ± 8.50 |
7.66 ± 0.57 |
0.002 |
Abbreviations: CBD, corticobasal degeneration; DLBD, dementia with Lewy body; MSA, multiple system atrophy; PSP, progressive supranuclear palsy; UPDRS, Unified Parkinson's Disease Rating Scale.
The study showed a significant change both in the PSP-CDS (initial 11.82 ± 1.47; final 14.64 ± 1.80; change 2.72 ± 1.84; p = 0.001) and the total UMSARS over 6 months (initial 43.2 ± 15.8; final 54 ± 20.58; change 6.0 ± 0.8; p = 0.001; Table 4).
|
Initial |
Final |
Change |
p-Value |
|
|---|---|---|---|---|
|
PSP-CDS |
11.82 ± 1.47 |
14.64 ± 1.80 |
2.72 ± 1.84 |
0.001 |
|
UMSARS |
43.2 ± 15.8 |
54 ± 20.58 |
6.0 ± 0.81 |
0.001 |
Abbreviations: CDS, Clinical Deficits Scale; MSA, multiple system atrophy; PSP, progressive supranuclear palsy; UMSARS, Unified MSA Rating Scale.
Among atypical parkinsonism, MSA patients have the highest mortality (Table 5; Fig. 3).
|
Disease |
Univariate analysis |
||
|---|---|---|---|
|
HR |
95% CI |
p-Value |
|
|
PSP |
– |
– |
– |
|
MSA |
3.5 |
0.31–0.38 |
0.306 |
Abbreviations: CI, confidence interval; MSA, multiple system atrophy; PSP, progressive supranuclear palsy; HR, hazards ratio.
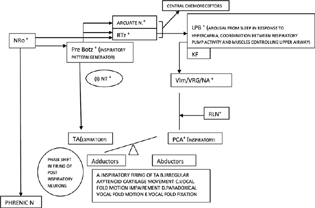
-
Fig. 3 Pathophysiology of respiratory manifestations in MSA. Arcuate N, Arcuate Nucleus; KF, Kolliker Fuse Nucleus; LPB, lateral parabrachial complex; m(I)NT, medullary inhibitory neurotransmitters; MSA, multiple system atrophy; NA, nucleus ambiguous; NRo, nucleus raphe obscureus; PCA, posterior cricoarytenoid; Pre Botz, pre Botzinger complex; RLN, recurrent laryngeal nerve; RTz, tetrotrapezoid body; TA, thyroarytenoid; VLM, ventrolateral medulla; VRG, ventral respiratory group. Note: The symbol “+” indicates affected/degeneration in MSA.
Fig. 3 Pathophysiology of respiratory manifestations in MSA. Arcuate N, Arcuate Nucleus; KF, Kolliker Fuse Nucleus; LPB, lateral parabrachial complex; m(I)NT, medullary inhibitory neurotransmitters; MSA, multiple system atrophy; NA, nucleus ambiguous; NRo, nucleus raphe obscureus; PCA, posterior cricoarytenoid; Pre Botz, pre Botzinger complex; RLN, recurrent laryngeal nerve; RTz, tetrotrapezoid body; TA, thyroarytenoid; VLM, ventrolateral medulla; VRG, ventral respiratory group. Note: The symbol “+” indicates affected/degeneration in MSA.
Discussion and Conclusion
The age at presentation was higher in DLBD and CBD patients (69 ± 5.8 and 67 ± 1.7 years, respectively) compared with MSA and PSP (61 ± 6.7 and 65 ± 3.3 years, respectively) patients. The male to female ratio was 2.7:1. Among PSP patients, PSP-RS was the most common type (58.33%) and MSA-C was more prevalent than MSA-P (71.44% versus 28.57%). A retrospective analysis of 334 PSP patients found that PSP-RS predominated (72%), followed by PSP-parkinsonism (13.5%).20 Our results are in agreement with other epidemiological studies in which the majority of MSA cases, ∼70–80%, are of MSA-C type in the Asian population.8
A high prevalence of NMS was seen. This is attributed to the involvement of multiple areas outside the basal ganglia early in the disease course. Our findings are in line with the results of the PRIAMO Study.1
A significant change in PSP-CDS and UMSARS was seen over 6 months (PSP-CDS: initial 11.82 ± 1.47, final 14.6 ± 1.80, change 2.72 ± 1.84, p = 0.001; UMSARS: initial 43.2 ± 15.8, final 54 ± 20.58, change 6.0 ± 0.81, p = 0.001). One study revealed that the PSP-CDS showed significant 12-month change (baseline 8.6 ± 3.6; follow-up 10.8 ± 3.6; annualized difference 3.4 ± 3.4; n = 49; p < 0.0001).21 Another study on 126 MSA patients showed a significant decline in UMSARS over 6 months (initial 51.2 ± 17; final 59.3 ± 17.7; change 9.2 ± 8.9).21
During the 6-month follow-up period, three patients died (two MSA and one PSP). Two MSA patients who reported stridor died suddenly. In a study of 21 MSA patients,22 the leading causes of death were cardiopulmonary arrest in 33.3%, urinary tract infections in 23.8%, wasting syndrome in 14.3%, and pneumonia in 14.3%. In IPD, infectious pneumonia and cerebrovascular accidents accounted for 33.3% and 14.3% of deaths, respectively. Several mechanisms for sudden death have been proposed in MSA patients: vocal cord abductor palsy23 24 25 (Fig. 4), sleep apneas, and minimal chemo-sensitivity to hypoxia (central and pulmonary chemoreflex circuitry; Fig. 1). The death of the PSP patient was due to a fall from a height resulting from postural instability. Hence, falls in PSP merit special attention. Falls are attributed to the prominent involvement of the indirect locomotor system and the pedunculopontine nucleus (PPN).26 Nonmedical approaches to reduce fall frequency include exercise training, physical therapy, and auditory and visual feedback. Cholinesterase inhibitors, coenzyme Q10, and DBS of PPN have shown promise in reducing fall frequency.26 In a cohort study, dysphagia-related deaths, which included aspiration pneumonia, sepsis related to total parenteral nutrition, and suffocation, accounted for 91% of deaths in the PSP population.27 Dysphagia in atypical parkinsonism may be related to the degeneration of the swallowing pattern generators in the medulla coupled with the disruption of the supra medullary influences (cortical, basal ganglia, and limbic) on them (Table 6).28
|
Symptoms |
Treatment |
|---|---|
|
Anxiety |
Cognitive behavioral therapy (CBT), mindfulness-based stress reduction, cognitive bias modification intervention, noninvasive brain stimulation, tDCS, DBS, buspirone29 |
|
Apathy |
Amantadine, SSRI (mirabegron, trazodone), cholinesterase inhibitors, GABA agonist (zolpidem), educational and behavioral interventions29 |
|
Depression |
SSRI, SNRI, MAOI, TCA, dopamine agonists, ECT/TMS, CBT29 |
|
Orthostatic hypotension |
Salt tablets, water intake (up to 2.5 L/day), acute water bolus drinking, physical counter maneuvers, abdominal binder, recumbent exercises, waist-high compression stockings (15–20 mm Hg pressure), midodrine, droxidopa, atomoxetine, fludrocortisone, pyridostigmine7 |
|
Urinary dysfunction |
Behavioral therapy, intermittent or permanent catheterization (if postvoid volume > 100 mL), antimuscarinics, mirabegron, desmopressin, tibial neuromodulation, onabotulinum injections, sacral neuromodulation, bladder augmentation, sacral deafferentation and anterior root stimulation7 |
|
Constipation |
Graded exercise, change In toileting position, abdominal massage, adequate fiber, probiotics, laxatives, prokinetics, suppositories12 |
|
Stridor |
NPPV/CPAP/tracheostomy7 |
|
Pain |
Botulinum injections (dystonic pain), levodopa/dopamine agonists (neuropathic pain)6 |
|
Dysphagia |
Modified diet, feeding tube, percutaneous gastrostomy, treatment of cervical dystonia29 |
|
Sleep disturbances |
RBD: safe sleeping environment, clonazepam, melatonin, gabapentin, sodium oxybate, zopiclone, temazepam7 EDS: modafinil, dextroamphetamine/methamphetamine9 |
Abbreviations: CPAP, continuous positive airway pressure therapy; DBS, deep brain stimulation; ECT, electroconvulsive therapy; EDS, excessive daytime sleepiness; GABA, gamma-aminobutyric acid; MAOI, monoamine oxidase-B inhibitors; NPPV, noninvasive positive-pressure ventilation; RBD, rapid eye movement sleep behavior disorder; SNRI, serotonin norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressants; tDCS, transcranial direct current stimulation; TMS, transcranial magnetic stimulation.

-
Fig. 4 Disruption of medullary reflexes in atypical parkinsonism. A 5, noradrenergic neurons of ventrolateral pons; Botc, Botzinger complex; CVLM, caudal ventrolateral medulla; DMV, dorsal motor nucleus of vagus; eSN, efferent sympathetic nerve; NA, nucleus ambiguous; PG SYM; postganglionic sympathetic; PN, phrenic nerve; Pre-Botc, pre-Botzinger complex; PVN, paraventricular nucleus; RTN/Pfrg, retrotrapezoid nucleus/parafacial respiratory group; RVLM, rostral ventrolateral medulla; Rvrg, rostral ventral respiratory group; SON, supraoptic nucleus; VN, vagus nerve; VRG, ventral respiratory group.
Fig. 4 Disruption of medullary reflexes in atypical parkinsonism. A 5, noradrenergic neurons of ventrolateral pons; Botc, Botzinger complex; CVLM, caudal ventrolateral medulla; DMV, dorsal motor nucleus of vagus; eSN, efferent sympathetic nerve; NA, nucleus ambiguous; PG SYM; postganglionic sympathetic; PN, phrenic nerve; Pre-Botc, pre-Botzinger complex; PVN, paraventricular nucleus; RTN/Pfrg, retrotrapezoid nucleus/parafacial respiratory group; RVLM, rostral ventrolateral medulla; Rvrg, rostral ventral respiratory group; SON, supraoptic nucleus; VN, vagus nerve; VRG, ventral respiratory group.
Atypical parkinsonian disorders are rare, and usually more severe than Parkinson's disease. These are often misdiagnosed as IPD in the early phases because of the symptom overlap, transient symptomatic improvement with levodopa, and lack of objective diagnostic biomarkers. However, the emergence of red flag signs ultimately provides a clue. Though no definite cure exists to date, symptomatic and supportive management should be optimized given the tremendous impact of various NMS on the quality of life and survival.
The limitations of the study include the small sample size of 27 patients and the use of subjective scales.
Conflict of Interest
None declared.
References
- Non-motor symptoms in atypical and secondary parkinsonism: the PRIAMO study. J Neurol. 2010;257(1):5-14.
- [Google Scholar]
- Separate neural representations of depression, anxiety and apathy in Parkinson's disease. Sci Rep. 2017;7(1):12164.
- [Google Scholar]
- Norepinephrine deficiency in Parkinson's disease: the case for noradrenergic enhancement. Mov Disord. 2014;29(14):1710-1719.
- [Google Scholar]
- Apathy in Parkinson's disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14(5):518-531.
- [Google Scholar]
- Prevalence of pain in atypical parkinsonism: a systematic review and meta-analysis. J Neurol. 2019;266(9):2093-2102.
- [Google Scholar]
- An update on MSA: premotor and non-motor features open a window of opportunities for early diagnosis and intervention. J Neurol. 2020;267(9):2754-2770.
- [Google Scholar]
- Nucleus of the solitary tract, medullary reflexes, and clinical implications. Neurology. 2017;88(12):1187-1196.
- [Google Scholar]
- Sleep disturbance in movement disorders: insights, treatments and challenges. J Neurol Neurosurg Psychiatry. 2021;92(7):723-736.
- [Google Scholar]
- Anatomy and physiology of normal sleep.Sleep and Neurologic Disease. Academic Press; 2017. p. :1-28. In: , chap. 1
- [CrossRef] [Google Scholar]
- Neurogenic bladder in progressive supranuclear palsy: a comparison with Parkinson's disease and multiple system atrophy. Neurourol Urodyn. 2018;37(5):1724-1730.
- [Google Scholar]
- Severe constipation in Parkinson's disease and in parkinsonisms: prevalence and affecting factors. Front Neurol. 2019;10:621-2019.
- [Google Scholar]
- Life expectancy of parkinsonism patients in the general population. Parkinsonism Relat Disord. 2020;77:94-99.
- [Google Scholar]
- Clinical diagnosis of progressive supranuclear palsy: the Movement Disorder Society criteria. Mov Disord. 2017;32(6):853-864.
- [Google Scholar]
- Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100.
- [Google Scholar]
- Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496-503.
- [Google Scholar]
- The Progressive Supranuclear Palsy Clinical Deficits Scale. Mov Disord. 2020;35(4):650-661.
- [Google Scholar]
- Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS) Mov Disord. 2004;19(12):1391-1402.
- [Google Scholar]
- Subtypes of PSP and prognosis: a retrospective analysis. Ann Indian Acad Neurol. 2021;24(1):56-62.
- [Google Scholar]
- The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12(3):264-274.
- [Google Scholar]
- Causes of death in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2007;78(3):327-329.
- [Google Scholar]
- Pathogenesis of laryngeal narrowing in patients with multiple system atrophy. J Physiol. 2001;536:237-249. (Pt 1):
- [Google Scholar]
- Vocal cord electromyographic correlates of stridor in multiple system atrophy phenotypes. Parkinsonism Relat Disord. 2020;70:31-35.
- [Google Scholar]
- Involvement of medullary serotonergic groups in multiple system atrophy. Ann Neurol. 2004;55(3):418-422.
- [Google Scholar]
- Falls in progressive supranuclear palsy. Mov Disord Clin Pract (Hoboken). 2019;7(1):16-24.
- [Google Scholar]
- Impact of aspiration pneumonia on the clinical course of progressive supranuclear palsy: a retrospective cohort study. PLoS One. 2015;10(8):e0135823.
- [Google Scholar]
- Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929-969.
- [Google Scholar]
- Update on therapeutic strategies for atypical parkinsonian syndromes. Turkish J Neurol. 2020;26:111-121.
- [Google Scholar]







