Translate this page into:
The evaluation of the clinical, laboratory and the radiological findings of the fifty-five cases diagnosed with tuberculous, Brucellar and pyogenic spondylodiscitis
Address for correspondence: Dr. Kadriye Kart Yasar, Department of Clinical Microbiology and Infectious Diseases, Haseki Training and Research Hospital, Adnan Adıvar Cad. Haseki, Aksaray, 34300, Istanbul, Turkey. E-mail: kadriyeyasar@hasekihastanesi.gov.tr
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
In this study, the evaluation of the clinical, laboratory and radiological findings belonging to 55 cases that were hospitalized in our clinic to be followed-up and were diagnosed with tuberculous, brucellar and pyogenic spondylodiscitis (SD) was aimed.
Materials and Methods:
The cases with SD were evaluated retrospectively. Hematological, serological, biochemical laboratory tests and imaging technics were used for diagnosis.
Results:
Of 55 cases aged ranging between 25 to 79, 33 (59%) were female. The cases with tuberculous SD (TBSD), brucellar SD (BSD) and pyogenic SD (PSD) were found in 24 (43%), 12 (21%) and in 19 (34%) patients. Erytrocyte sedimentation rate, increased C-reactive protein, and leucocytosis were present in 51 (91%), 22 (39%) and 8 (14%) cases. The number of the cases with history of previous surgery or trauma was 14 (25%). Diagnosis of TBSD was established by acid fast bacilli positiveness and Löwenstein Jensen culture positiveness, in two and seven patients, respectively. While all 12 cases with BSD had positive standard tube aglutination test, only 3 (25%) had hemoculture positivity. In PSDs, diagnosis was confirmed with culture positivity in 9 of 19 cases.Of the cases in our study, 89% responded to medical treatment while three required surgery and three died (5.5% and 5.5%, respectively).
Conclusion:
SD may develop secondary to infections or following spinal surgical procedures and traumas. Also, the importance of endemicity should be kept in mind, beside the helpful diagnostic findings while treatment regulation.
Keywords
Brucellosis
pyogenic spondylodiscitis
spondylodiscitis
tuberculosis
vertebral osteomyelitisIntroduction
Introduction
The vertebral infections, that have been first described by Hippocrates, make up 2-4% of all osteomyelitis cases.[1] Lumbar and thoracal vertebrates are the most frequently involved vertebral regions.[2] The increased incidence of the vertebral infections has recently been noted, due to the prolonging of average age, malnutrition, immunodeficiency, diabetes mellitus, drug abuse, the increasing use of endovascular and genitourinary devices, human immunodeficiency virus(HIV), septicemia, and the chronic use of steroids.[3] Spondylodiscitis (SD) may be complicated due to the epidural, paravertebral or psoas abscess. Since brucellosis and tuberculosis are prevalent in our country, these two infections must be taken into account in the differential diagnosis of the vertebral osteomyelitis cases, and related laboratory tests must be performed. If SD is seen in young patients, rare non-infectious causes of spondylodiscitis should be considered. Seronegative spondyloarthritis is a general term for a group of joint conditions including ankylosing spondylitis, reactive arthritis (eg, Reiter syndrome), psoriatic arthritis, arthritis associated with inflammatory bowel disease (eg, Crohn disease or ulcerative colitis), and undifferentiated spondyloarthritis. Inflammatory involvement of the intervertebral disks by spondyloarthritis is known as SD or Andersson lesion. Rheumatic SD is a noninfectious condition that occurs in about 8% of patients with ankylosing spondylitis.[4]
The diagnosis in SD is generally established by the increased erythrocyte sedimentation rate (ESR), leukocytosis, the increased C-reactive protein (CRP) and typical radiological findings in addition to the clinical picture. The blood cultures and microbiological and histopathological investigation of the biopsy samples obtained from the lesion is the gold standard in diagnosis of SD.[5–7] The rare appearance of the disease in addition to non-typical symptoms and the findings in early stages lead to delayed diagnosis, progressive forms and complications.
In this study, the evaluation of the clinical, laboratory and radiological findings belonging to 55 cases that were hospitalized in our clinic, to be followed-up and diagnosed with tuberculous, brucellar and pyogenic SD, was aimed.
Materials and Methods
The cases that applied to our clinic with complaints of prolonged dorsalgia and fever, and hospitalized with diagnosis of SD have been evaluated retrospectively. The diagnosis was established by performing biochemical tests and blood count, CRP, ESR beside the specific tests for ethiological pathogens such as Rose Bengal test, STA test, Ziehl–Neelsen(ZN) staining, L-J culture, non-specific culture, hemoculture, biopsy and histopathological investigation, and imaging methods such as computed tomography (CT) and magnetic resonance imaging (MRI). The patients, who were administered non-specific antibiotic treatment until the establishment of the diagnosis, received tuberculosis or brucellosis treatment according to the clinical, laboratory and radiological findings pointing out specific SD. The cases with PSD, BSD and TBSD have been treated for at least 6 weeks, 12 weeks and 12 months, respectively.
Findings
Of 55 cases aged ranging between 25 to 79, 33 (59%) were female. The cases with TBSD, BSD and PSD were found in 24 (%43), 12 (21%) and in 19 (34%) patients. The 49 (88%) patients had dorsalgia whereas fever was present in only 16 (29%) patients. ESR, increased CRP, and leukocytosis were present in 51 (91%), 22 (39%) and 8 (14%) cases. The number of the cases with history of previous surgery or trauma was 14 (25%). Of our cases, 14% had comorbid chronic diseases such as predominantly renal failure and diabetes. The diagnosis of TBSD was established by acid fast bacilli (AFB) positiveness and L-J culture positiveness, in two and seven patients, respectively. While all 12 cases with BSD had positive STA test, only 3 (25%) had hemoculture positivity. Methicillin resistant Staphylococcus aureus in four cases, methicillin sensitive S.aureus in two cases, methicillin resistant S. epidermidis,Streptococcus intermedius and Enterobacter cloacae were isolated from patients in each one of them as the responsible pathogen in PSDs. According to the radiological imaging studies, paravertebral abscess, psoas and epidural abscesses were found in seven, five and two cases as complication of SD (31% in total). Spinal abscess was determined in six PSD and TBSD cases and three BSD cases; hence, frequency of paraspinal abscess showed no difference according to etiological pathogen [Figures 1–3]. Comorbid meningitis, iliac osteomyelitis and iliapsoas abscess were found in nine, three and three of the cases with TBSD, respectively. Of the four cases, three (5.5%) required surgical treatment and no complications developed. Only three (5.5%) cases with TBSD died, despite the therapy and three recovered with sequelae (5.5%).
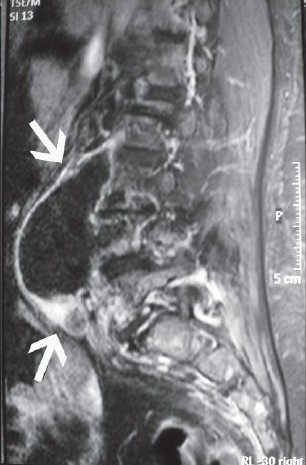
- Abscess, lying along the right psoas muscle due to pyogenic spondylodiscitis caused by Streptococcus intermedius in T1 weighted sagittal magnetic resonance imaging scan

- Tuberculosis-induced vertebral destruction with diffuse paravertebral abscess in vertical magnetic resonance imaging scan

- Contrast enhanced T1W sagittal magnetic resonance imaging images at level C7 show intramedullary expansile spinal tuberculoma with peripheric enhancement and spondylodiscitis between T12-L1
Discussion
Spinal infections can be described etiologically as pyogenic, granulomatous (tuberculous, brucellar, fungal) and parasitic.[8] SD, a term including vertebral osteomyelitis, spondylitis and discitis, besides being a factor of severe morbidity because of the neurological sequelae, is an important health problem due to the administration of parenteral antibiotic during long-period hospitalization, bed occupation in service, high cost and invasive diagnostic investigations, and requirement of surgical treatment.[8]
Vertebral osteomyelitis generally develops via a haematogenic invasion from an infectious source in a different region of the body. The infection spreads to the avascular intervertebral discs and the neighboring vertebrates, originating from the vertebrate matter.[9] Most cases in our study were localized in the lumbar and thoracic regions in compliance with the literature.[10] Its insidious beginning and silent clinical course lead to delayed diagnosis. Approximately 30% of the SD sareiatrogenic.[91112] In some patients, a medical history of blunt trauma to the spinal column or invasive spinal procedures may be elicited.[913] Of the PSD cases and granulomatous SD cases,around 58% and 11% had medical history of trauma or spinal surgical intervention, respectively.
Approximately 37% of spontaneous PSD will not have an definitesource.[14] The common organisms including Staphylococcus aureus and streptococcus species and Gram-negative bacilli as intravenous drug users are frequently isolated. Stapylococcus species were the responsible pathogen in seven of the nine cases with PSD. In our study, no pathogenic or suspicious origin was determined in 30% of the SD cases even by the invasive diagnostic tests. In developed countries, Mycobacterium tuberculosis, fungal infections and parasitic infestations are common in immunosupressve patients.[15–17] In the countries with prevalent tuberculosis and brucellosis such as our country, granulomatous SDs cases are of the major clinical forms.[1318–21] In our study, PSD rate is also significant (36%). However, many of PSD cases are followed up by neurosurgeons and orthopedists in our hospital. Only PSD cases which applied to our department, and were given medical treatment without surgery, were included in this study. Therefore, the actual numbers may not have been recorded. The medical history of previous operation and trauma in more than half of our cases with PSD supports the data associated with frequency of the post operative SD cases.[22]
The diagnostic criteria of SD are imaging findings compliant with spondylitis accompanied by spinal pain, fever and sensitivity, therefore MRI is quite useful in the differentiation of PSD and TBSD. Radiologically, intervertebral disc space narrowing, osteolysis in the vertebral “endplates” or corpus, decreased signal activity in the intervertebral disc space with T1-weighted sequences and increased with T2-weighted sequences and the contrast involvement in MRI imaging is considered as SD.[1023] Of the all SD cases, 25% is associated with epidural abscess.[16] In our study, 26% of the cases revealed paraspinal abscess formation, and only in two cases were epidural localized. There were no significance in ratio among three types of SD in terms of abscess complication. The dominant clinical symptom was the increased mechanical dorsalgia with movement and it was present in 88% of our cases, accompanied by limited motion.[1516]
The essential bases of SD treatment composed of medication, immobilization, use of orthez and surgical debridment, therefore the treatment should be multidisciplinary. Primarily, medical therapy according to the responsible pathogen is a proper approach. However, an indication for surgical intervention would be available in clinically larger abscesses nonresponsive to medical therapy, cases with neurological deficit due to pressure of spinal cord, remarkable vertebral destruction, deformity and stability loss.[24] The prognosis is generally good in SDs.[182425] TBSD was present in the two cases of death, and the diagnosis was proven by culture positiveness. TBSD is generally known to have a good prognosis. However, mortality and complication risks increase, particularly in immuncompromised cases or cases with comorbid diseases or advanced age.[13]
As a conclusion, SD may develop secondary to infections, or following spinal surgical procedures and traumas. Also, the importance of endemicity should be kept in mind, beside the helpful diagnostic findings during treatment regulation.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: A descriptive and comperative study of 219 cases. Ann Rheum Dis. 1997;56:709-15.
- [Google Scholar]
- Evaluation of conservative treatment of non specific spondylodiscitis. Eur Spine J. 2009;18(Suppl 1):S143-50.
- [Google Scholar]
- Spinal changes in patients with spondyloarthritis: Comparison of MR imaging and radiographic appearances. Radio Graphics. 2005;25:559-70.
- [Google Scholar]
- Vertebral osteomyelitis at a Norwegian university hospital 1987-97: Clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998;30:147-51.
- [Google Scholar]
- Diagnostic value of image guided fine needle aspiration cytology in assessment of vertebral and paravertebral lesions. J Cytol. 2007;24:79-81.
- [Google Scholar]
- Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668-79.
- [Google Scholar]
- Spondylodiscitis: Update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11-24.
- [Google Scholar]
- Spondilodiscitis after transvaginal oocyte retrieval for in vitro fertilisation. Acta Orthop Belg. 2005;71:249-51.
- [Google Scholar]
- Postoperative spondylodiscitis: Etiology, clinical findings, prognosis, and comparison with nonoperative pyogenic spondylodiscitis. Clin Infect Dis. 1999;29:339-45.
- [Google Scholar]
- Favorable outcome of long-lasting thoracic spondylodiscitis with spinal epidural abscess induced by Staphylococcus aureus. Southern Med J. 2003;96:70-3.
- [Google Scholar]
- Spinal tuberculosis (Pott's disease): Its clinical presentation, surgical management, and outcome. A survey study on 694 patients. Neurosurg Rev. 2001;24:8-13.
- [Google Scholar]
- Surgical treatment results of spinal tuberculosis with combined anterior and posterior approach. J Med Sci. 2007;7:790-6.
- [Google Scholar]
- A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect. 2007;55:158-63.
- [Google Scholar]
- Lumbar and lumbosacral tuberculous spondylodiscitis in adults: Redefining the indications for surgery. J Bone Joint Surg. 2002;84:530-4.
- [Google Scholar]
- Pyogenic spondylodiscitis after percutaneous endoscopic lumbar discectomy. J Korean Neurosurg Soc. 2010;48:455-60.
- [Google Scholar]
- Vertebral osteomyelitis in northern Spain: Report of 62 cases. Clin and Exper Rheum. 1999;17:447-52.
- [Google Scholar]






