Translate this page into:
The effect of vitamin A supplementation on stimulated T-cell proliferation with myelin oligodendrocyte glycoprotein in patients with multiple sclerosis
Address for correspondence: Prof. Aliakbar Saboor-Yaraghi, Assistant Professor of Biochemistry, Department of Nutrition and Biochemistry, School of Public Health, Tehran University of Medical Sciences, Tehran, Postal code - 141613151, Iran. E-mail: asaboor@sina.tums.ac.ir
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Multiple sclerosis (MS) is an autoimmune disease whereby myelin sheath of the central nervous system is destroyed. Vitamin A is known to play a role in the immune system. It has been recognized that some metabolites of vitamin A can be used effectively to treat experimental autoimmune encephalomyelitis (EAE).
Aims:
The effect of vitamin A as retinyl palmitate on T-cell proliferation in MS patients.
Setting and Design:
This study is a double blind clinical trial of two test groups over a period of 6 months.
Materials and Methods:
Thirty five multiple sclerosis (MS) patients were divided into two groups. One group received 25,000 IU/day vitamin A (as retinyl palmitate) and the other group were administered a placebo. The peripheral blood mononuclear cells (PBMCs) were separated and stimulated with myelin oligodendrocyte glycoprotein (MOG) and phytohemagglutinin (PHA) before and after the trial period. BrdU calorimetric assay was performed to measure cell proliferation.
Statistical Analysis:
Analysis of covariance (ANCOVA) and paired t-test were used to analyze the data.
Results:
Observations showed statistical significant differences in the reduction of cell proliferation in the presence of MOG and fetal calf serum (FCS) in the culture medium, between patients receiving vitamin A and the placebo (P = 0.046). Although, this difference was not significant between the two vitamin A and placebo groups in MOG treatment with human serum, a decrease was observed in the group of patients taking vitamin A supplements (P = 0.019). Phytohemagglutinin did not cause any change in cell proliferation between the two groups.
Conclusion:
The results suggest supplementation with retinyl palmitate in patients with MS reduce MOG stimulatory effects on T-cells.
Keywords
Cell proliferation
multiple sclerosis
myelin oligodendrocyte glycoprotein
vitamin A
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease and major disorder of the central nervous system (CNS), which usually begins between the ages of 20 to 40 years.[1] The exact etiology of MS remains unknown but there is strong evidence to support the theory that autoimmune mechanisms such as Th1 like response from myelin specific CD4+ T-cells are considered a part of the pathophysiology of this disease.[23] The main event in the pathogenesis of MS is myelin sheath destruction. Therefore, proteins in the myelin of the CNS are likely candidates to be involved in the pathogenic self- response.[4]
Myelin oligodendrocyte glycoprotein (MOG) is one of a few autoantigens on the surface of the myelin sheath that is readily available to autoreactive T-cells and because MOG is the only protein that is expressed in CNS, the response of T-cells likely plays an important role in the pathogenesis of MS.[4]
Additionally, vitamin A plays an important role in the immune system and retinoids have been identified as having many functions that regulate immune responses.[5] Retinoids, metabolites of vitamin A and ligands of its steroid receptor superfamily are useful in the treatment of experimental autoimmune encephalomyelitis (EAE).[6] Experimental autoimmune encephalomyelitis is an animal model of MS and occurs by immunization with myelin antigens such as myelin basic protein (MBP), proteolipid protein (PLP) or MOG.[7–12] The studies that were conducted on retinoic acid (RA) make the assumption that vitamin A may perform a direct immune function through its effect on mitogenic adjustment signals in T-cells.[13]
Retinyl palmitate is a retinol ester that can be converted to RA in the body.[14] According to the positive impact of vitamin A on the immune system as well as on EAE, the animal model, and as a high level of RA is potentially toxic, this study aimed to examine the potential of vitamin A (as retinyl palmitate) supplement to reduce a mitogenic response of MOG as an autoantigen in patients with MS.
Materials and Methods
Participants
Thirty six relapsing-remitting MS (RRMS) patients consented to participate in this survey (written consent was obtained from all of them); they were under the guidance of two neurologists, (one subject withdrew for personal reasons in mid-project, so 35 people remained until the end of the study). The Ethics Committee of Tehran University of Medical Sciences approved the study. All patients used Interferon beta-1a (Avonex) as treatment. This study was a randomized double blind clinical trial with vitamin A (as retinyl palmitate) and placebo. A general questionnaire was provided for each patient that set out an explanation of the essential information about vitamin A supplementation. The supplements were taken for a period of 6 months as 25,000 IU retinyl palmitate (Zahravi, Tabriz, Iran) per day, to this way that they had been given either vitamin A or placebo for 3 months and after this period, their liver function and clinical signs were checked and either vitamin A or placebo was given to them for a further 3 months if no problems were diagnosed.
Sample blood collection
Blood samples were collected from the subjects in sterile tubes with Heparin as the anticoagulant. 1 ml of blood samples were saved in a sterile microtube without any anticoagulant for serum blood isolation. Peripheral blood mononuclear cells (PBMCs) were isolated on Ficoll-Histoprep gradients (BAG Health Care GmbH, Germany). Cells were counted and diluted to 1 × 106 cells per ml culture medium (RPMI 1640; PAA laboratories GmbH, Austria). 500 μl of this cell dilution was separated and 10% fetal calf serum (FCS; Gibco, Invitrogen, UK) was added to the remaining cells. 75,000 PBMC were cultured in 96-well flat-bottomed microtiter plates (Nunc, Thermo Fisher Scientific Inc, Denmark) in the presence of 5 μl/ml MOG [(35-55) human; Anaspec, USA]. 75,000 PBMCs from diluted cells without FCS were cultured in the presence of MOG as above and then 10 μl of serum from coagulated blood was added to these cells. 75,000 cells were left stimulated with 5 μl/ml phytohemagglutinin (PHA; Sigma, USA) and untreated. Cells were incubated at 37 °C in a 5% CO2 incubator for 96 h. After this period, a cell proliferation ELISA BrdU kit (Roche Diagnostics GmbH, Germany) was used for cell proliferation assessment. Briefly BrdU was added to each well and incubated for 4 h in the same incubator. After this period, the next stages were performed according to company protocol. The absorbance was measured at 450 nm using ELISA plate reader. The design of study has been summarized in a flow chart [Figure 1].
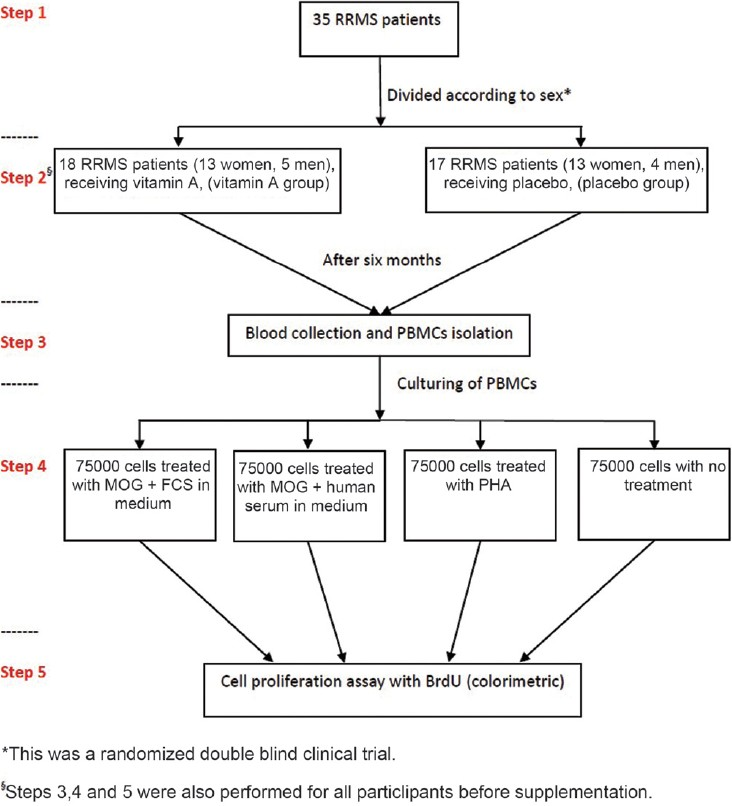
- Study design to determine the effects of vitamin A on cell proliferation in RRMS patients
Statistical analysis
Data were analyzed using SPSS version 17.0 for windows. Analysis of covariance (ANCOVA) was used to test the main and interaction effects of categorical variables on a continuous dependent variable. Data taken from each group was compared by paired-sample T-test. P-value less than 0.05 was considered statistically significant.
Results
The thirty five subjects in this study were divided into two groups: the vitamin A group (18 patients) and the placebo group (17 patients). 74.3% of participants were female and 25.7% were male. The ratio of women to men was approximately 2.9. The mean age was 32 years. Table 1 shows the profile of patients participating in the study.
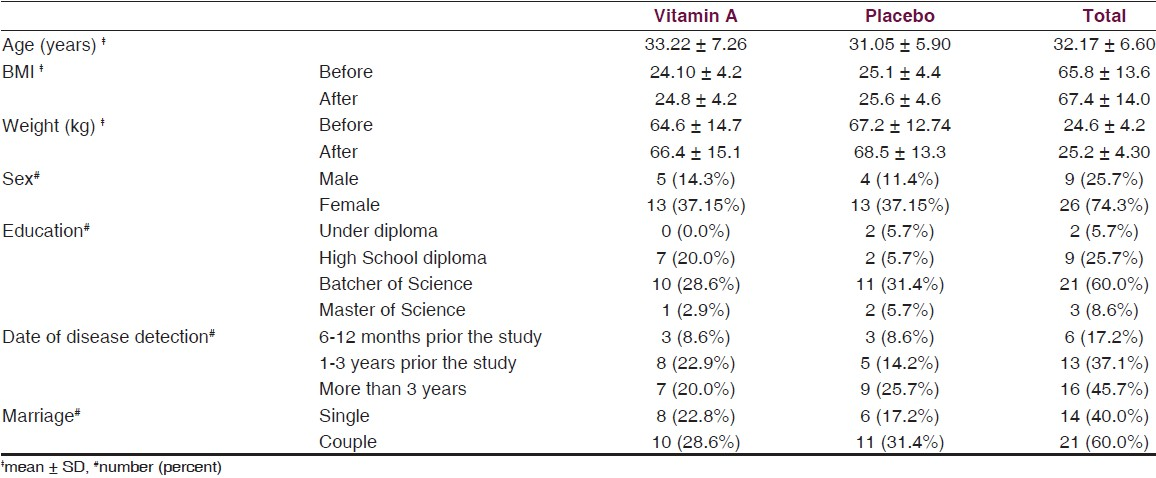
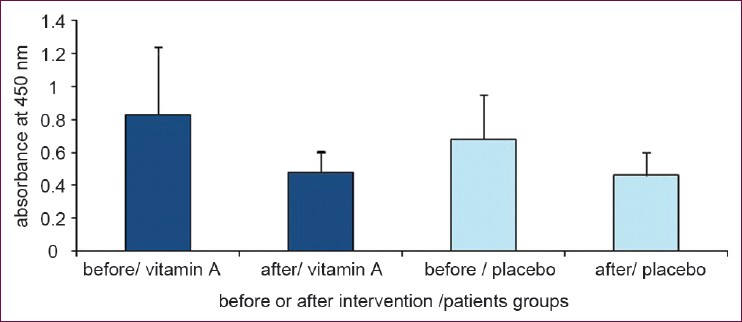
In Figure 2, results of cell stimulation with MOG are shown. From two different treatments of MOG, the treatment that contained the fetal calf serum (FCS) showed significant difference between the group treated with vitamin A and the placebo group (P = 0.046), as determined with ANCOVA statistical analysis [Figure 2a].
- (a) Cell proliferation with MOG in the presence of FCS in culture medium in the two groups; the group receiving vitamin A supplement and that which received the placebo. (b) Cell proliferation with MOG in the presence of his/her blood serum (Data are presented as mean ± SD)
In another assay MOG with human serum was used to stimulate cells, but no statistically significant differences were observed in stimulated cells between the vitamin A treated group and the placebo group (P = 0.21, ANCOVA). This indicated that the proliferation of cells treated with MOG and human serum has been decreased in both vitamin A and placebo groups. Although, the reduction in cell proliferation occurred in both groups after the supplementation period, this reduction in the group receiving vitamin A had a statistically significant difference compared with before of supplementation (P = 0.019 and P = 0.054 in vitamin A and placebo groups respectively; analyzed by paired sample T-test) [Figure 2b]
Results of stimulated cell proliferation with PHA are shown in Figure 3 and reveals no differences between the two groups before and after 6 months supplementation period (P = 0.22; analyzed by ANCOVA; P = 0.84 and P = 0.9 in vitamin A and placebo groups respectively; analyzed by paired sample T-test). Cells cultured without mitogen treatment showed no difference in proliferation between the two groups (P = 0.47 analyzed by ANCOVA) [Figure 4].
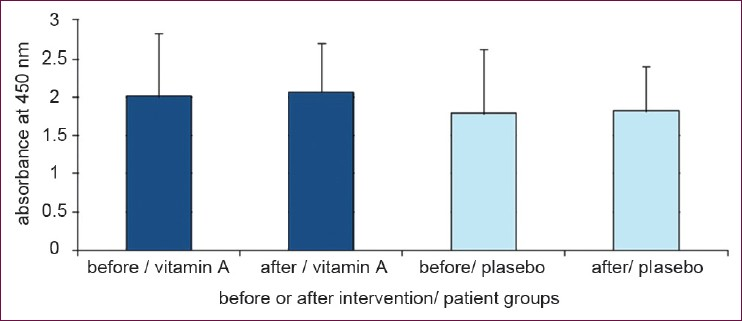
- Cell proliferation in the presence of PHA in culture medium. Retinyl palmitate could not change T-cell proliferation in presence of PHA (Data are presented as mean ± SD)
- Cell proliferation without any treatment. No differences have been obtained in two groups who receiving vitamin A supplement and placebo. (Data are presented as mean ± SD)
Discussion
Myelin oligodendrocyte glycoprotein is a membrane glycoprotein with 218 amino acids from a superfamily of immunoglobulin that has a lower amount than other myelin proteins and is located in the outer surface of the oligodendrocyte membrane. This location makes it directly accessible to antibodies and is therefore a target for immune responses in MS.[1] Among other myelin antigens such as MBP or PLP, MS patient's lymphocytes are the most responsive to MOG.[15] In this study, it has been demonstrated that a vitamin A supplement taken by people with relapsing-remitting MS was able to reduce lymphocyte proliferation stimulated by MOG compared with those that received placebo.
The ability of vitamin A to control growth and differentiation is dependent on the metabolism of retinol to RA.[1617] In the first step alcohol dehydrogenases (ADH) converts retinol to retinaldehyde; the next step is oxidation of retinaldehyde to RA by retinaldehyde dehydrogenases (RALDH). Retinoic acid acts as a ligand for RA receptor signaling.[1617] Many studies have indicated that vitamin A regulates the secretion of IL-10 from Th2 cells. IL-10 can inhibit the synthesis of IL-2 inflammatory cytokine from Th1 cells.[18] According to reports, RA can stimulate the proliferation of normal peripheral lymphocytes mediated IL-2.[13] Two consecutive signals are required for resting lymphocyte proliferation. First, the antigenic stimulation of TCR/CD3 complex leads the cell cycle from a quiescent state to the G1 phase;[1920] secondly IL-2 binding to its receptor drives the G1 phase to S, so that proliferation occurs.[21] It has been shown that RA can reduce the production of IL-2[182223] through its direct effect on IL-2 gene expression,[24] suggesting that reduction in cell proliferation stimulated by MOG in this study could be due to reduced production of IL-2.
Further studies on cell proliferation under the stimulation of MBP in vitro showed reduced proliferation in a dose-dependent procedure of all trans-RA (ATRA).[6] In another study, different concentrations of ATRA on MBP-specific lymph node cell proliferation in SJL/J mice stimulated with MBP revealed that ATRA inhibited T-cell response to MBP in a dose-dependant manner.[22] The effect of 13-cis-RA on lymphocyte proliferation (without the presence of a specific antigen) after oral administration to Lewis rats showed that when serum levels of 13-cis-RA reached the desired level, lymphocyte proliferation responses in these animals decreased.[25] In another survey on MS patients, lymphocyte proliferation was studied by adding RA dissolved in DMSO to a culture medium and no changes were observed in proliferation.[26] This study revealed that no significant difference in cell proliferation was stimulated with PHA and without any treatment between two groups, that which received vitamin A and that which received placebo, perhaps due to an only moderating effect of vitamin A on mitogenic reactions influenced by MOG antigens in these patients.
Conclusion
The results show vitamin A effects on the proliferation of PBMCs stimulated with MOG but no effect on the proliferation of PBMCs stimulated with PHA. The vitamin A effect on MOG can decrease the activity of this specific antigen on T-cell stimulation in vitro.
This research has been supported by Tehran University of Medical Sciences and Health Services grant (88-03-27-9576). The authors are very grateful for all colleagues in the Department of Nutrition and Biochemistry, School of Public Health, Tehran University of Medical Sciences and Iranian Center for Neurological Research for their kind helps and advices in the laboratory. We would like to thank all the patients for their kind collaboration in this study.
The final version of the manuscript has been approved by all authors and each author believes that the manuscript represent honest work.
Source of Support: Nil
Conflict of Interest: there are no known conflicts of interest associated with this publication
References
- New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291-301.
- [Google Scholar]
- In vitro Th2 deviation of myelin-specific peripheral blood lymphocytes from patients with multiple sclerosis. J Neuroimmunol. 2006;171:156-62.
- [Google Scholar]
- Antigen-specific T cells in autoimmune diseases with a focus on multiple sclerosis and experimental allergic encephalomyelitis. Cell Mol Life Sci. 1999;56:5-21.
- [Google Scholar]
- Regulation of FoxP3+ regulatory T cells and Th17 cells by retinoids. Clin Dev Immunol. 2008;2008:1-12.
- [Google Scholar]
- Retinoic acid promotes the development of Th2-like human myelin basic protein-reactive T cells. Cell Immunol. 2002;215:54-60.
- [Google Scholar]
- The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579-621.
- [Google Scholar]
- The immunopathogenesis and regulation of T-cell-mediated demyelinating disease. Immunol Today. 1994;15:356-61.
- [Google Scholar]
- A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: Fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol. 1995;25:1951-9.
- [Google Scholar]
- Evolution of the T-cell repertoire during the course of experimental immune-mediated demyelinating disease. Immunol Rev. 1995;144:225-44.
- [Google Scholar]
- Delineation of the minimal encephalitogenic epitope within the immunodominant region of myelin oligodendrocyte glycoprotein: Diverse V beta gene usage by T cells recognizing the core epitope encephalitogenic for T cell receptor V betab and T cell receptor V betaa H-2b mice. In: Eur J Immunol. Vol 26. 1996. p. :2470-9.
- [Google Scholar]
- Acceleration of experimental autoimmune encephalomyelitis in interleukin-10-deficient mice: Roles of interleukin-10 in disease progression and recovery. Cell Immunol. 1998;188:118-24.
- [Google Scholar]
- All-trans retinoic acid stimulates IL-2-mediated proliferation of human T lymphocytes: Early induction of cyclin D3. J Immunol. 2006;177:2851-61.
- [Google Scholar]
- Estimating the potential for vitamin A toxicity in women and young children. J Nutr. 2002;132:2907S-19S.
- [Google Scholar]
- Reactivity to myelin antigens in multiple sclerosis.Peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J Clin Invest. 1993;92:2602-8.
- [Google Scholar]
- Families of retinoid dehydrogenases regulating vitamin A function: Production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315-24.
- [Google Scholar]
- Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem Biol Interact. 2003;143-4:201-10.
- [Google Scholar]
- Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446-64.
- [Google Scholar]
- Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994;14:4889-901.
- [Google Scholar]
- Commitment point during G0 → G1 that controls entry into the cell cycle. Mol Cell Biol. 2003;23:2351-61.
- [Google Scholar]
- Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol. 1995;54:450-8.
- [Google Scholar]
- Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006;7:27.
- [Google Scholar]
- Retinoic acid-induced transcriptional modulation of the human interferon-γ promoter. J Biol Chem. 1996;271:26783-93.
- [Google Scholar]
- Immunosuppressive activity of 13-cis-retinoic acid and prevention of experimental autoimmune encephalomyelitis in rats. J Clin Invest. 1991;88:1331-7.
- [Google Scholar]
- All-trans retinoic acid potentiates the ability of interferon Beta-1b to augment suppressor cell function in multiple sclerosis. Arch Neurol. 1998;55:315-21.
- [Google Scholar]






