Translate this page into:
Synchronous presentation of prolactinoma and supratentorial tanycytic ependymoma
*Corresponding author: Visvanathan Krishnaswamy, Department of Neurosurgery, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India. visvanathan.k@sriramachandra.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Nair JN, Bathala R, Krishnaswamy V, Mahadevan S. Synchronous presentation of prolactinoma and supratentorial tanycytic ependymoma. J Neurosci Rural Pract. 2024;15:140-2. doi: 10.25259/JNRP_217_2023
Abstract
Tanycytic ependymomas mostly occur in the spinal cord and it is the rarest histological subtype of ependymoma. A 29-year-old male was referred from the infertility clinic after serum prolactin levels were found to be elevated. Magnetic resonance imaging (MRI) brain showed an irregular necrotic lesion in the periventricular region of the left parietal lobe which had an intraventricular component and associated perilesional edema. In addition, a sellar mass with suprasellar extension was also found on the MRI. He was started on cabergoline therapy for macroprolactinoma and underwent a left parietal craniotomy, and microsurgical excision of the tumor using intraoperative neurosonographic guidance. Histologically, the tumor showed spindle cytologic features and poorly developed inconspicuous pseudorosettes, with areas of rounded nuclear profiles and perinuclear cytoplasmic clearing. Tumor cells were positive for vimentin, glial fibrillary acidic protein and S100, and negative for epithelial membrane antigen. Ki67 was <7%. He was diagnosed with tanycytic ependymoma and a coexistent prolactinoma. He received 10 cycles of image-guided radiotherapy. Post-operative imaging showed minimal residual tumor the size of which remained stable at 1-year follow-up scan. The pituitary macroadenoma regressed with cabergoline therapy and he clinically improved. This presentation of synchronous macroprolactinoma and tanycytic ependymoma has not been reported in the literature previously. An exhaustive literature review showed only 18 previously reported cases of supratentorial tanycytic ependymoma.
Keywords
Craniotomy
Cabergoline
Prolactinoma
Tanycytic ependymoma
Synchronous intracranial tumors
INTRODUCTION
In 1978, Friede and Pollak first described the term “tanycytic ependymoma,” the least frequently encountered histological variant of ependymoma which is seen predominantly in the spinal cord. It was recognized as a separate pathological entity in the third edition of the World Health Organization (WHO) Central Nervous System (CNS) five classifications.[1,2] Its atypical histological features may make it challenging to diagnose, as it may resemble schwannomas or pilocytic astrocytomas.[3]
Ependymoglial cells, also called tanycytic glia or tanycytes, are primitive long bipolar cells in the circumventricular organs and the central canal.[2,4] In light microscopy, the spindle morphology of the cells is seen (like schwannoma cells) with uniformly arranged cells and oval nuclei. Pilocytic astrocytoma has long processes that resemble tanycytic ependymoma. The lack of Rosenthal fibers, compact perivascular arrangement of the cells, and presence of large nuclei differentiate them from pilocytic astrocytoma.[4,5] Pathologists rely on immunohistochemical and histopathological characteristics to make the final diagnosis. Tanycytic ependymoma shows glial fibrillary acidic protein (GFAP) and vimentin positivity but is not S-100 positive. Schwannomas are S-100 positive and GFAP negative. Pilocytic astrocytoma is GFAP positive, but vimentin negative.[6] These WHO grade II tumors carry a relatively better prognosis than other grade II ependymomas.[5] More than 90% of sellar masses and 10–15% of all intracranial neoplasms are pituitary adenomas. The coexistence of a pituitary adenoma with other intracranial tumors is not uncommon; however, the real incidence is unknown. No syndromic associations have been identified incorporating these two lesions.[7]
CASE REPORT
A 29-year-old gentleman presented with reduced libido and erectile dysfunction of around 1-year duration. He had no history of severe headache or visual disturbance. On clinical examination, he was well virilized (Axillary hair Tanner stage 3 and pubic hair Tanner stage 5 with a testicular volume of 20 mL each). There was no goiter and system examination was normal. Biochemical evaluation showed hyperprolactinemia (Prolactin level 458 ng/mL) and other anterior pituitary hormonal profile was normal. Magnetic resonance imaging brain revealed a well-defined hyperintense lesion of size 17 × 15 × 18 mm in the sellar-suprasellar region with a displacement of the infundibulum to the right. Areas of blooming were noted with no diffusion restriction. The lesion was seen to indent the cavernous part of the left internal carotid artery (25% encasement). Another 3.5 × 1.9 × 3.2 cm well-demarcated lesion was seen in the periventricular region of the left parietal lobe with a small intraventricular component along the posterior horn of the left lateral ventricle [Figure 1]. An 18F-fluorodeoxyglucose (FDG) Positron emission tomography–computed tomography showed a hypermetabolic soft-tissue density lesion in the sella and an FDG AVID periventricular mass lesion in the left parietal lobe with a small intraventricular component and significant surrounding edema raising the possibility of ependymoma or glioblastoma. The patient was initiated on cabergoline therapy for the prolactinoma and underwent a left parietal craniotomy and microsurgical excision of the left parietal lobe lesion. The lesion was reached by a trans-sulcal superior parietal lobule approach guided by intraoperative ultrasound. The tumor was moderately vascular, grayish brown, and firm in consistency. It was involving the body of the lateral ventricle and extending above into the parietal white matter. Few areas of calcification were noted peroperatively. The tumor was debulked using cavitron ultrasonic aspirator (CUSA). There were no perioperative complications and the patient had an uneventful postoperative recovery. Histopathological examination showed spindle cytologic features and poorly developed inconspicuous pseudorosettes, with areas of rounded nuclear profiles and perinuclear cytoplasmic clearing. Immunohistochemical staining revealed strong GFAP positivity with scattered S-100 positivity, confirming tanycytic ependymoma. Tumor cells showed vimentin positivity and were EMA negative with Ki 67 of <7%. Post-operative imaging showed a minimal residual tumor in the left parietal periventricular region for which patient received 10 cycles of image guided radiotherapy. Two-year follow-up imaging showed regression of the prolactinoma and no progression of the ependymoma [Figure 2] with a return of normal sexual function and serum prolactin level had fallen to 32.2 ng/mL at the time of review.
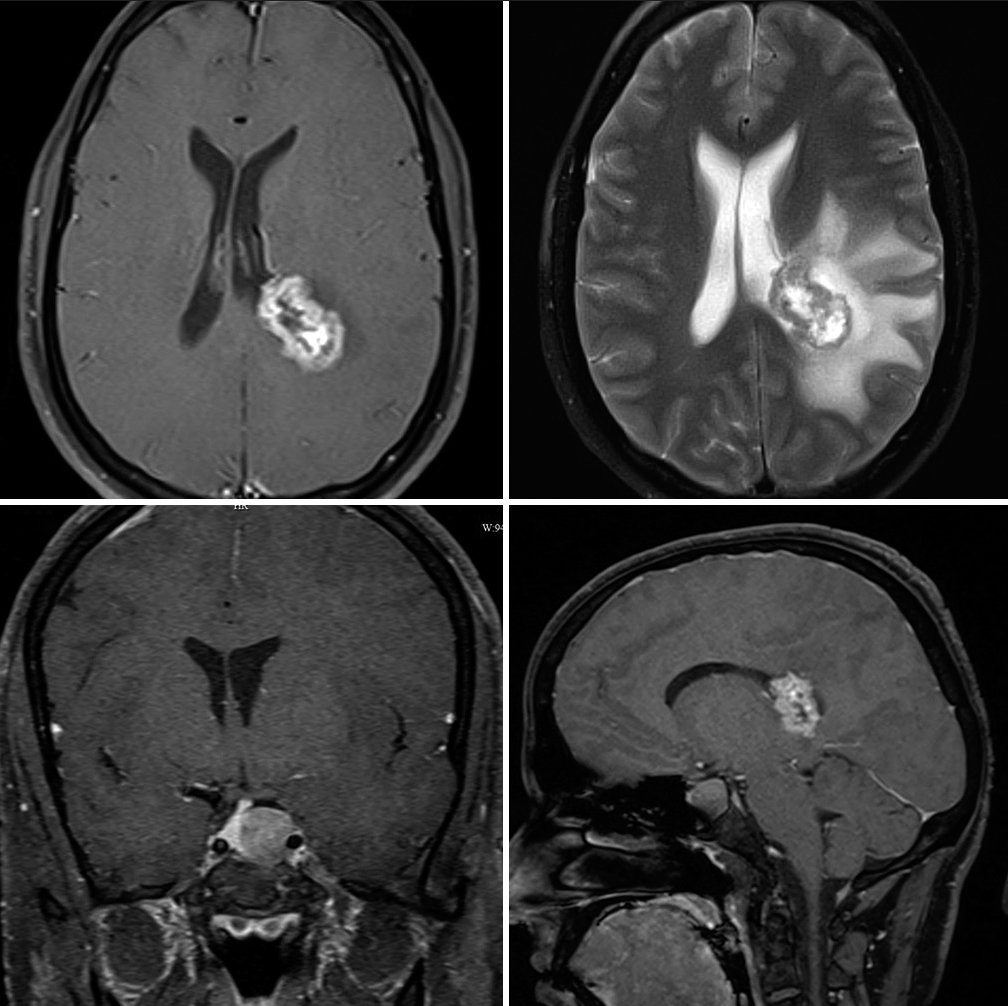
- Pre-operative axial contrast-enhanced magnetic resonance imaging and T2 images (top) and coronal and sagittal cuts (bottom) showing synchronous right parietooccipital enhancing lesion and pituitary macroadenoma.
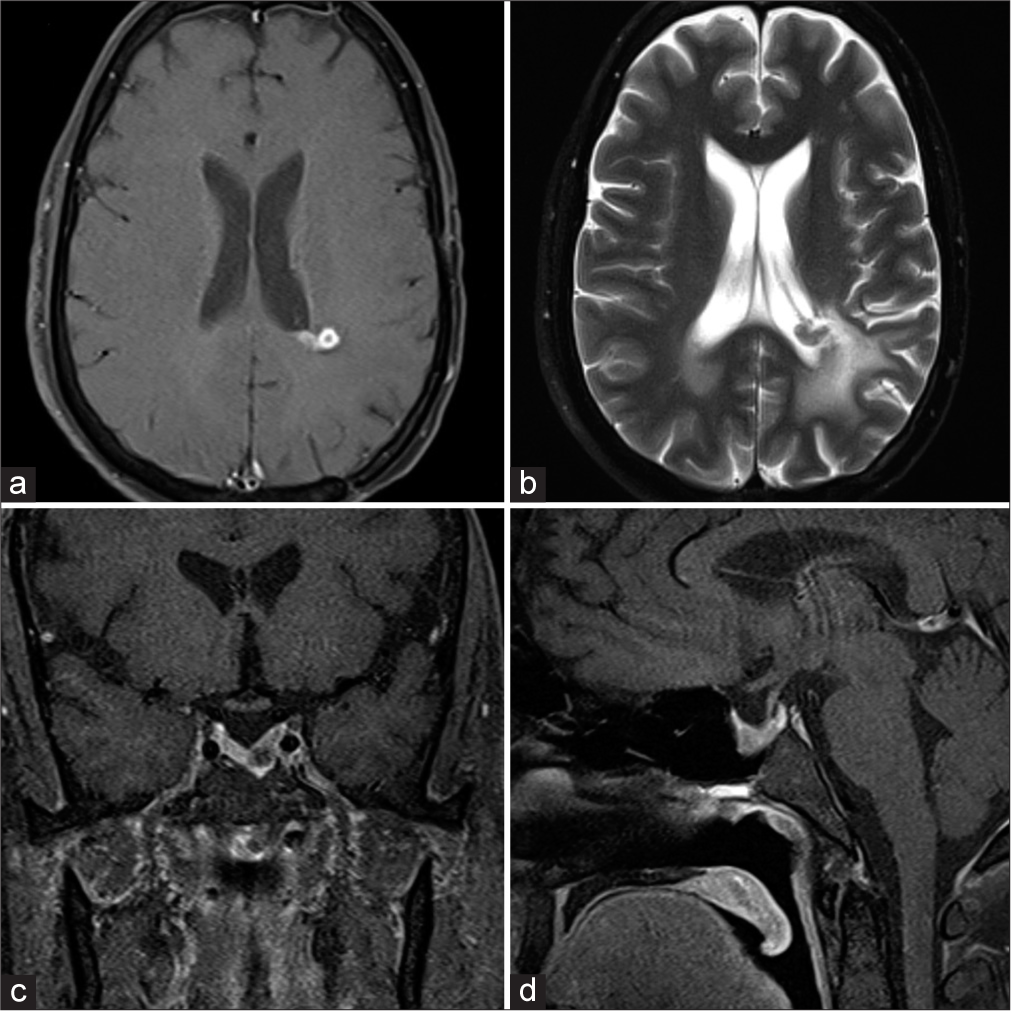
- (a-d) Post-operative contrast-enhanced and T2-weighted magnetic resonance imaging showing residual ependymoma and resolving prolactinoma.
DISCUSSION
Only 18 cases of supratentorial tanycytic ependymoma have been reported (10 ventricular and eight subcortical). They have been reported to occur in the 4th–6th decades of life with male predominance and are typically smaller than 4 cm and are usually solid or cystic in consistency with calcification.[8,9] Radiologically, these tumors appear hypointense to isointense on T1-weighted images, non-enhancing to heterogeneously enhancing with contrast, and minimal peritumoral edema on fluid attenuated inversion recovery images.[9] Follow-up data from nine of the 18 cases suggests a favorable prognosis. The longest follow-up period was 3 years and none of them had any other intracranial or systemic malignancy. The new 2021 WHO classification of CNS tumors defines two molecular subtypes of supratentorial ependymoma – ZFTA and YAP1 fusion. Tanycytic morphological variant is no longer listed as one of the subtypes of ependymoma and is included in the histological description of ependymoma in the new integrated, layered reporting system.[10]
Occurrence of simultaneous distinct intracranial neoplasms is rare in the literature and the commonly reported association has been that of meningioma with glioma. Pituitary adenomas have been reported to occur in association with meningiomas, medulloblastomas, neurinomas, craniopharyngiomas, astrocytomas, and gliomas.[11]
One case report of simultaneous occurrence of prolactinoma with ependymoma of the fourth ventricle has been published; however, this was not of tanycytic morphology. The occurrence of two distinct neoplasms in the same patient raises questions about the underlying molecular mechanisms involved in intracranial neoplastic processes.[7]
It is worth noting that both pituitary adenomas and ependymomas are known to have over-expression of genes on chromosomes 17 and 19, but no syndromic association between the two has been described in the literature. In addition, two distinct neoplasms may share genetic alterations on the same chromosome, which could explain their synchronous occurrence.[11]
There may be a population of patients with coexisting tumors who remain undiagnosed due to benign nature of many of these lesions. Prolactin, a hormone known to play a role in cell growth and anti-apoptosis, has been implicated in driving neoplasia of various organs, including the breast, genitourinary system, and hematopoietic system. However, it is not currently known whether hyperprolactinemia plays a role in the growth of ependymomas.[12]
CONCLUSION
As our understanding of molecular mechanisms underlying intracranial neoplastic processes improves, the study of simultaneous intracranial neoplasms may shed light on previously unknown molecular mechanisms involved in these diseases. The increasing availability of molecular diagnostic capabilities is likely to facilitate such studies and may lead to improved treatment options and outcomes for patients with these rare and complex conditions.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803-20.
- [CrossRef] [PubMed] [Google Scholar]
- The cytogenetic basis for classifying ependymomas. J Neuropathol Exp Neurol. 1978;37:103-18.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative diagnosis of tanycytic ependymoma: Pitfalls and differential diagnosis. Diagn Cytopathol. 2001;24:289-92.
- [CrossRef] [PubMed] [Google Scholar]
- Tanycytic ependymoma: A challenging histological diagnosis. Case Rep Neurol Med. 2013;2013:170791.
- [CrossRef] [PubMed] [Google Scholar]
- Spinal tanycytic ependymoma with hematomyelia--case report--. Neurol Med Chir (Tokyo). 2005;45:168-71.
- [CrossRef] [PubMed] [Google Scholar]
- Prolactinoma associated with an ependymoma in the fourth ventricle: A case report and review of the literature. Oncol Lett. 2015;10:228-32.
- [CrossRef] [PubMed] [Google Scholar]
- A case of tanycytic ependymoma arising from the cerebral hemisphere. Brain Tumor Pathol. 2006;23:91-5.
- [CrossRef] [PubMed] [Google Scholar]
- Features of intraventricular tanycytic ependymoma: Report of a case and review of literature. Int J Clin Exp Pathol. 2014;7:3399-407.
- [Google Scholar]
- The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021;23:1231-51.
- [CrossRef] [PubMed] [Google Scholar]
- Coexisting intracranial tumors with pituitary adenomas: Genetic association or coincidence? J Can Res Ther. 2010;6:221-3.
- [CrossRef] [PubMed] [Google Scholar]
- Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol Metab. 2002;13:245-50.
- [CrossRef] [PubMed] [Google Scholar]







