Translate this page into:
Symptomatic Bilateral Xanthogranuloma of the Choroid Plexus
Address for correspondence: Dr. Selin Tural Emon, Tibbiye Cad No: 40 Uskudar, 34668 Istanbul, Turkey. E-mail: turalselin@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Xanthogranulomas (XGRs) of the choroid plexus are rare, asymptomatic, and benign lesions usually found incidentally. Here, we present a case of a 47-year-old male with bilateral XGR of the choroid plexus with periventricular edema and discuss our case in relation to a review of existing literature pertaining to the radiology of XGRs. To the best of our knowledge, this is the first reported case of bilateral trigonal XGR causing brain edema without ventricular dilatation. Despite the fact that they can cause hydrocephalus, XGRs are silent and benign lesions. Although the etiopathology of XGRs remains poorly understood, enhanced imaging analyses may provide additional information regarding edema and focal white matter signal changes.
Keywords
Choroid plexus
edema
headache
ventricle
xanthogranulomas
INTRODUCTION
Xanthogranulomas (XGRs) are benign lesions and rarely involve the central nervous system. They mostly occur in the choroid plexus of the lateral ventricles and third ventricle although they have also been detected in a number of other intracranial locations such as the suprasellar cistern, petrous apex, middle ear, and paranasal sinuses.[1] XGR is known as cholesterol granuloma and is a rare granulomatous tumor characterized by cholesterol clefts, hemosiderin deposits, lymphocytic infiltration, and fibrous proliferation.[123] XGRs are idiopathic, and usually asymptomatic, with an incidence at autopsy of approximately 1.6%–7.0%.[2] The prevalence of XGRs appears to peak at 20–40 years of age.[123] The majorities of symptomatic cases concern the choroid plexus of the third ventricle and predominantly lead to obstructive hydrocephalus.[3]
CASE REPORT
A 47-year-old male was admitted to our hospital with a 3-day history of sudden-onset severe headache. There was no reported history of trauma, and neurological examinations and fundoscopy were normal. He had no headache attacks, vertigo, or vomiting in his medical history. Routine physical and laboratory examinations were also normal.
Radiology
The first cranial computerized tomography scan showed bilaterally hyperdense lesions in the trigone of the lateral ventricles, with periventricular vasogenic edema on the left side. The left-sided and right-sided lesions were determined to be 13 mm × 12 mm × 8 mm and 11 mm × 8.5 mm × 7 mm in size, respectively [Figure 1a].
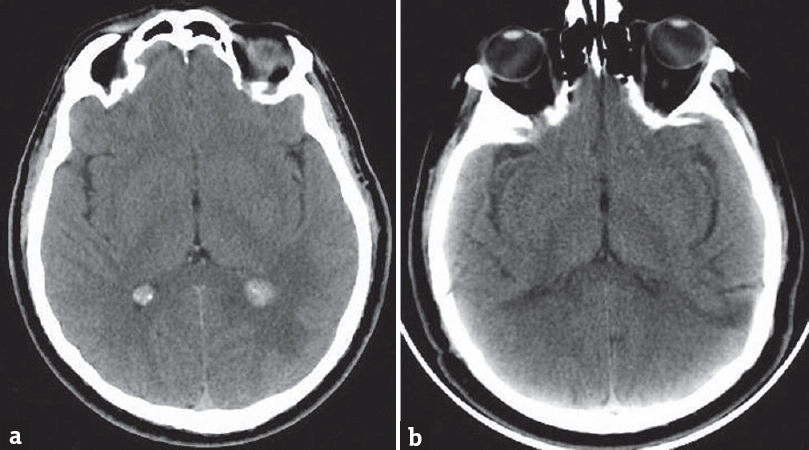
- (a) Cranial computed tomography scan showing bilateral hyperdense lesions in the trigone of the lateral ventricles along with periventricular vasogenic edema on the left side. (b) Postoperative control cranial computerized tomography
The first cranial magnetic resonance images (MRIs) revealed that the lesions were well-defined, isointense in T1 sequences and hypointense in T2 images. Following gadolinium administration, the images showed partial enhancement. Fluid attenuation inversion recovery images revealed edema surrounding the lesion in the left lateral ventricular area but without ventricle dilatation [Figure 2]. MR perfusion showed high relative cerebral blood volume and relative cerebral blood flow values in the lesions, whereas MR spectroscopy failed to reveal signs of malignancy. Positron emission tomography-CT scans also failed to reveal a primary lesion.
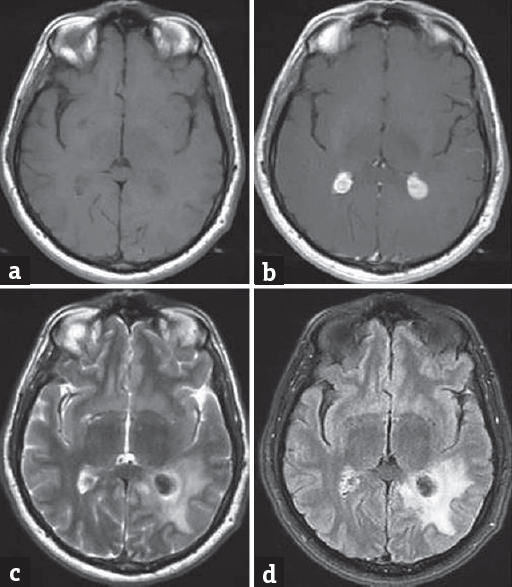
- (a-c) Cranial magnetic resonance imaging showing that the lesions were well-defined and isointense in T1 sequences and hypointense in T2 images. Following gadolinium administration, the lesions showed partial enhancement. (d) Fluid attenuation inversion recovery images showing edema around the lesion in the left lateral ventricular area without ventricle dilatation
Surgical treatment
First, we performed left occipital stereotactic craniotomy and completely excised the lesion, which was encapsulated, pale yellow, and elastic. Approximately 6 months later, the patient again complained of a headache. Control cranial MRI revealed edema around the lesion located within the right lateral ventricular area, along with postoperative changes on the left side [Figure 3]. Because of the surrounding edema, the lesion on the opposite side was also excised using the same surgical approach. Control cranial CT confirmed that the lesions had been completely excised and postoperative changes [Figure 1b]. Following surgery, postoperative examinations were normal and the patient's headache stopped.
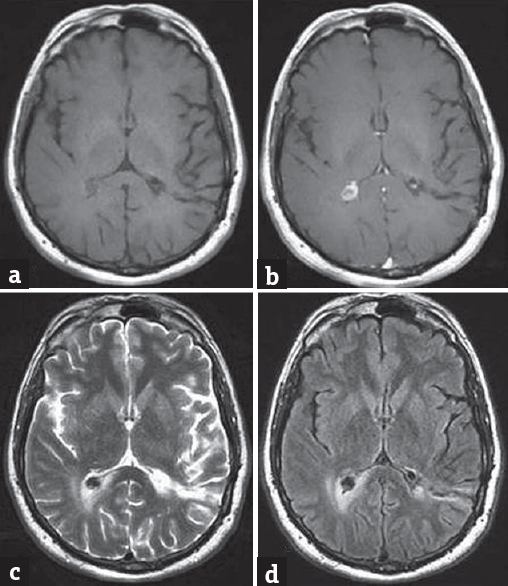
- (a-d) Six months after the first operation, cranial magnetic resonance imaging showing postoperative changes on the left side along with new edema surrounding the lesion in the right lateral ventricular area
Histopathology
Pathological evaluation of both excised lesions revealed hypocellular tissue with fibrosis and a composition of lamellated cholesterol clefts surrounded by multinucleated giant cells, lymphoid cells, histiocytosis, and calcifications. Histopathological diagnosis was, therefore, XGR of the choroid plexus [Figure 4].
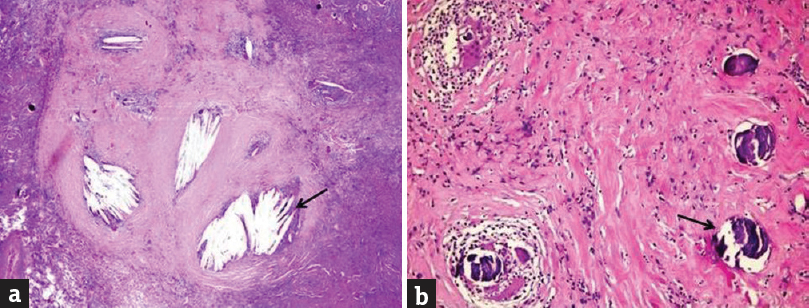
- (a) Hypocellular tissue with fibrosis and cholesterol clefts (black arrow) surrounded by multinucleated giant cells and a large number of inflammatory cells (H and E, ×100). (b) Hypocellular tissue with extensive lamellated calcification (black arrow), (H and E, ×200)
DISCUSSION
The first description of an XGR was reported by Blummer in 1900.[3] In general, XGRs are asymptomatic lesions and are usually discovered incidentally. XGRs can cause obstructive hydrocephalus, and patients may develop neurological symptoms such as a headache, papilledema, gait instability, cognitive impairment, and urinary disturbances.
XGRs, which are sometimes also referred to as cholesterol granulomas, are most commonly found as smooth, pale-yellow masses, which are occasionally encapsulated.[3] In our present case, the lesion was quite firm, well-circumscribed, and easily separated from surrounding tissue. XGRs represent tissue reactions with a pseudotumor aspect and are composed of cholesterol clefts and inflammatory cells such as foamy cells, histiocytes, multinucleated foreign body giant cells, hemosiderin deposits, and fibrous proliferation.[4] The secondary changes such as intralesional hemorrhage, fibrosis, or focal calcifications can also occur.
The etiology of XGRs remains controversial, although some existing reports relate the condition to an inflammatory response to the proliferation and invagination of the choroid plexus and neuroepithelial cyst transformation, and other reports suggest a developmental origin or high levels of lipids in the blood.[356] Laboratory blood examinations for our present patient showed that the amount of serum cholesterol was within the normal range. Furthermore, there were no associated systemic findings, such as superficial cutaneous xanthomas.
CT density of XGRs varies from hypodense to hyperdense states in comparison to brain tissue. On MRI, XGRs usually appear isointense or hypointense on T1-weighted images and hypointense on T2-weighted images and show variable enhancement following gadolinium administration. However, radiological features are very inconsistent because of the heterogeneity of XGR content. The presence of focal calcifications is reflected by hypointensity on T2-weighted images.[3] Therefore, differential diagnosis with other masses of the lateral ventricle, such as meningioma, papilloma, ependymal, and arteriovenous malformations, remains difficult.[7] According to cranial MRI findings, differential diagnoses for our case could have included intraventricular meningioma, pilocytic astrocytoma, papilloma of the choroid plexus, and metastases. We did not consider pilocytic astrocytoma or papilloma and carcinoma of the choroid plexus as realistic diagnoses as these are usually seen in patients of much younger age. In cases not known to have primary cancer, XGRs are thought to become a benign lesion. There was no marked limitation of diffusion in the diffusion images obtained in our patient; thus, there was little likelihood of metastasis. For the differential diagnosis, the findings in the present case were compatible with bilateral intraventricular meningioma. As a result, the presence of edema in our present case forced us to make a diagnosis and excise the lesion although no signs of intracranial hypertension at neurological and fundoscopic examinations. Existing literature shows that XGR usually leads to ventricle dilatation, except in two specific cases. First, Miranda et al.[3] reported XGR in the third ventricle with surrounding edema of the brain and hydrocephalus. Second, Kadota et al.[7] reported a case of left trigonal XGR with slightly dilated ventricles and hyperintense foci on T2-weighted images in the tegmentum of the pons and middle cerebellar peduncles. These changes in focal white matter signal were probably related to XGR and supported by Hokezu et al.’s[8] description of a similar case as a diagnosis of cerebrotendinous xanthomatosis. This was the first case of bilateral trigonal XGR causing brain edema but without ventricular dilatation.
Brain edema occurs as a result of the accumulation of excess water in the brain parenchyma. The blood–brain barrier (BBB) protects the brain's interstitial space from plasma extravasation under normal conditions. However, brain tumors cause vasogenic edema which disrupts the BBB. Abnormal vessels within the tumor, and the vessels in nearby tumor tissue, are thus affected by infiltrating tumor cells and show ultrastructural changes, such as elongated junctional clefts and an increase in the density of endothelial vesicles.[9] These changes have also been observed when vessels are not affected by tumor cells and occur because of the action of cytokines, which spread into peritumoral tissue and disrupt normal vascular morphology.[9] In our case, vasogenic edema may have led to the release and widespread distribution of cytokines. The development of edema on the right side 6 months after the first surgery was particularly notable in this respect.
CONCLUSION
Usually, XGRs are silent, benign lesions but are known to cause hydrocephalus. However, the etiopathology of XGR is poorly understood. Consequently, additional findings from imaging studies may provide important additional evidence such as edema and changes in signals arising from focal white matter. In some unusual cases, XGRs do not lead to hydrocephalus and instead cause perilesional edema, which surgeons should take into consideration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Calcified suprasellar xanthogranuloma presenting with primary amenorrhea in a 17-year-old girl: Case report and literature review. World Neurosurg. 2015;84:866.e11-4.
- [Google Scholar]
- Xanthogranuloma of the choroid plexus of the third ventricle: Case report and literature review. Neurocirugia (Astur). 2005;16:518-22.
- [Google Scholar]
- Pathological anatomy of tumors of the third ventricle. Neurochirurgie. 2000;46:257-67.
- [Google Scholar]
- Giant bilateral xanthogranulomas in a child: Case report. Neurosurgery. 1992;31:114-7.
- [Google Scholar]
- Symptomatic xanthogranuloma of choroid plexus with unilateral hydrocephalus. Case report. J Neurosurg. 1991;75:324-7.
- [Google Scholar]
- MR of xanthogranuloma of the choroid plexus. AJNR Am J Neuroradiol. 1996;17:1595-7.
- [Google Scholar]
- Cerebrotendinous xanthomatosis: Cranial CT and MRI studies in eight patients. Neuroradiology. 1992;34:308-12.
- [Google Scholar]






