Translate this page into:
Susceptibility or chemical shift artifacts in intracranial lipomas mimicking hemorrhage – Case series with review of the literature
*Corresponding author: Dr. K. Nagarajan, Department of Radio-Diagnosis, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India. lknagarajan1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kashyap R, Swathi Kiran R, Shivakrishna S, Nagarajan K. Susceptibility or chemical shift artifacts in intracranial lipomas mimicking hemorrhage – Case series with review of the literature. J Neurosci Rural Pract 2022;13:759-63.
Abstract
Intracranial lipomas are rare congenital malformations which are present in choroid plexus or subarachnoid locations along the corpus callosum and cisterns. Most of them are identified incidentally during neuroimaging studies done for other indications. Sometimes, they may be associated with other anomalies such as agenesis of corpus callosum and vascular malformations. In magnetic resonance imaging (MRI), they may be associated with chemical shift artifact (CSA) due to their lipid content and can mimic other more serious intracranial lesions. This effect seen in gradient echo MRI sequences can also be used to confirm the presence of these lesions. We report the imaging findings of six patients with intracranial lipomas that showed this chemical shift artifact with a review of the literature.
Keywords
Intracranial lipomas
Chemical shift artifact
Susceptibility artifact
Magnetic resonance imaging
INTRODUCTION
Intracranial lipomas are congenital malformations due to abnormal development of meninx primitive filling the subarachnoid cisterns that get resorbed during embryogenesis.[1] Earlier theories suggested that intracranial lipomas developed from hypertrophy of meningeal fatty tissues. The accepted etiology at present is the abnormal differentiation of the persistent meninx primitiva. Meninx primitiva refers to the mesenchymal covering of the brain from which the arachnoid mater, pia mater, and dura mater develop. Most intracranial lipomas are incidentally detected and are usually asymptomatic. The reported incidence is <1% of all intracranial tumors.[2] Pericallosal lipomas, the common type, account for 50% of all intracranial lipomas. Pericallosal lipomas may be associated with choroid plexus lipomas of the lateral ventricle. It has been hypothesized that the primitive meninx follows the choroidal fissure into the lateral ventricle, thereby leading to the development of choroid plexus lipomas.[3] The other common sites of intracranial lipomas include quadrigeminal cistern, suprasellar/interpeduncular, cerebellopontine angle, and sylvian cisterns. Few reports mention about association with corpus callosum agenesis and other midline structure defects. In MRI, intracranial lipomas show an interesting finding which is called as the chemical shift or susceptibility artifact.[4-6] We report a series of six cases of intracranial lipomas and discuss the possible reasons behind this finding of susceptibility effect that can potentially confuse with other lesion, but also may help to identify the fatty nature of these lesions.
CASE DETAILS
The clinical and imaging features of cases with intracranial lipomas are summarized in [Table 1]. The age group range of the cases was 12–48 years with two males and four females. Four patients presented with headache, one was asymptomatic, and one patient had pain over the left half of face and neck. The size of the lesion varied from 2 to 13 mm except for the pericallosal lipoma. The lipomas were also located in the following locations – pericallosal, suprasellar cistern, left quadrigeminal plate cistern, cistern of velum interpositum, right fornix, and right choroid plexus of lateral ventricle. Predominantly, the lesions were nodular with two curvilinear in morphology. CT showed peripheral calcifications in two of the seven lesions. Nodular and oval lesions showed complete chemical shift/susceptibility effect in gradient echo (GRE) T2* weighted images, while the curvilinear lesions showed peripheral blooming [Figures 1-6]. One of the pericallosal lipomas was associated with partial agenesis of rostrum and genu of corpus callosum. The 12-year-old boy had previous history of trauma for which CT showed fat-density along fornix and there were no traumatic lesions. Although he was asymptomatic, MRI was done to allay parents’ anxiety. With follow-up on out-patient (second and fifth patients) basis and telephonically, all of them are asymptomatic.
| S. No. | Age/Sex | History | Location of the | Size | Morphology | Calcification on CT | GRE T2* Susceptibility artifact |
|---|---|---|---|---|---|---|---|
| 1 | 46/F | Headache | Suprasellar cistern | 5×5 mm | Oval | - | Nodular |
| 2 | 48/F | Left face and neck pain | Left ambient cistern | 7×10×11 mm | Nodular | No | Nodular |
| 3 | 12/M | Asymptomatic | Right fornix | 4×3 mm 2×2 mm |
Nodular | No | Nodular |
| 4 | 39/F | Headache | Choroid plexus | 12×11 mm | Irregular | Nodular peripheral | Peripheral |
| 5 | 45/M | Headache | Pericallosal | 53×18 mm | Curvilinear | Peripheral | Peripheral |
| 6 | 13/F | Headache | Cistern of velum interpositum | 13×5 mm 4×3 mm |
Curvilinear and nodular | No | Peripheral |
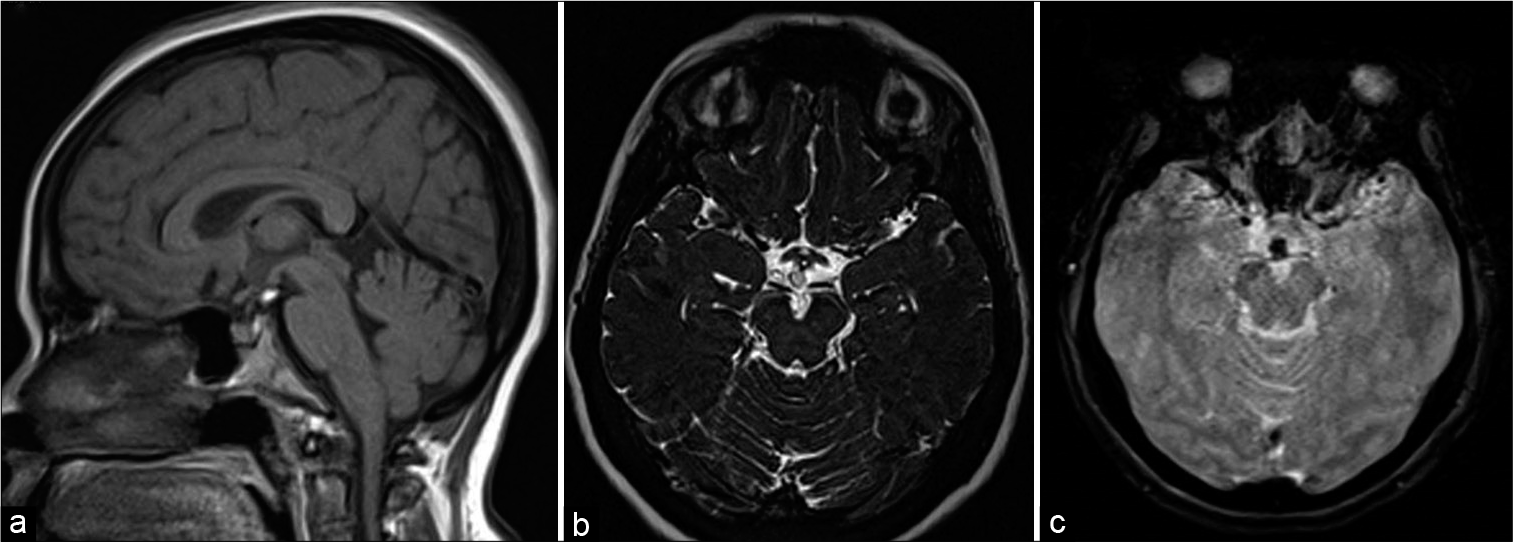
- A 46-year-old female with headache. T1-weighted sagittal (a), heavily T2-weighted SPACE axial (b), and gradient-echo GRE T2* axial images showing small speck of fat in suprasellar cistern just posterior to infundibular stalk showing susceptibility effect in (c).
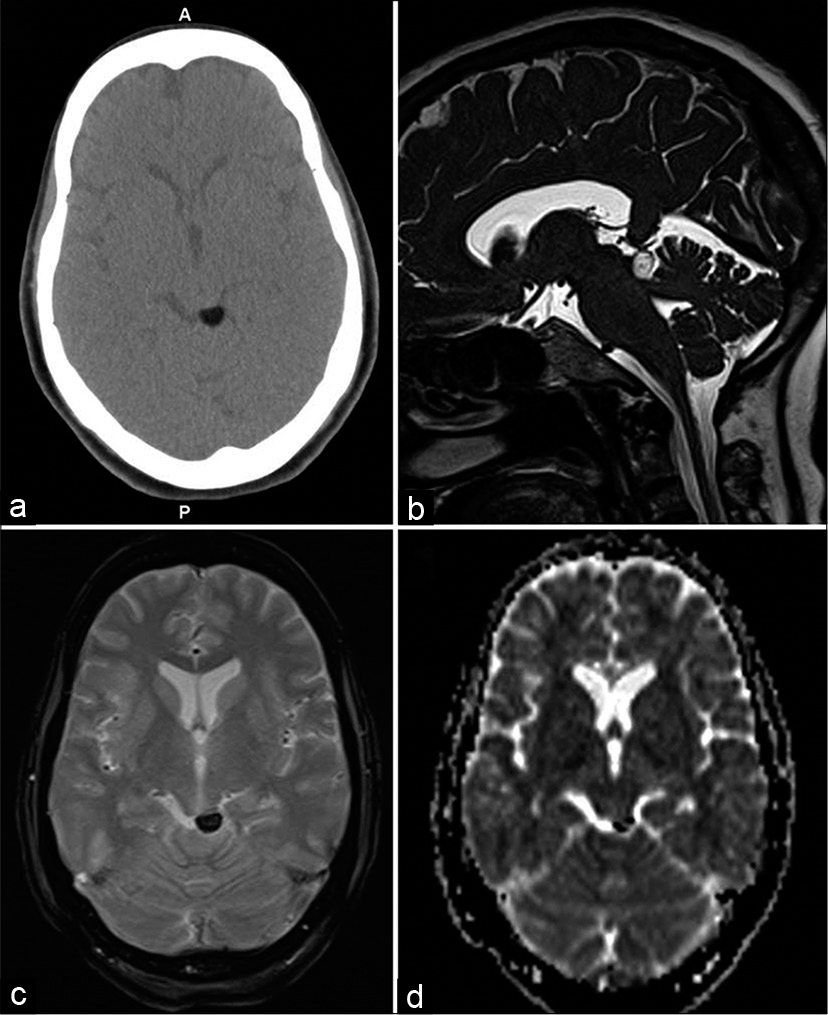
- A 48-year-old female with the left facial and neck pain. Axial CT (a), heavily T2-weighted SPACE sagittal (b), gradient-echo GRE T2* axial (c), and diffusion ADC image (d) showing focal lipomas in left quadrigeminal plate cistern with susceptibility effect in (c and d).
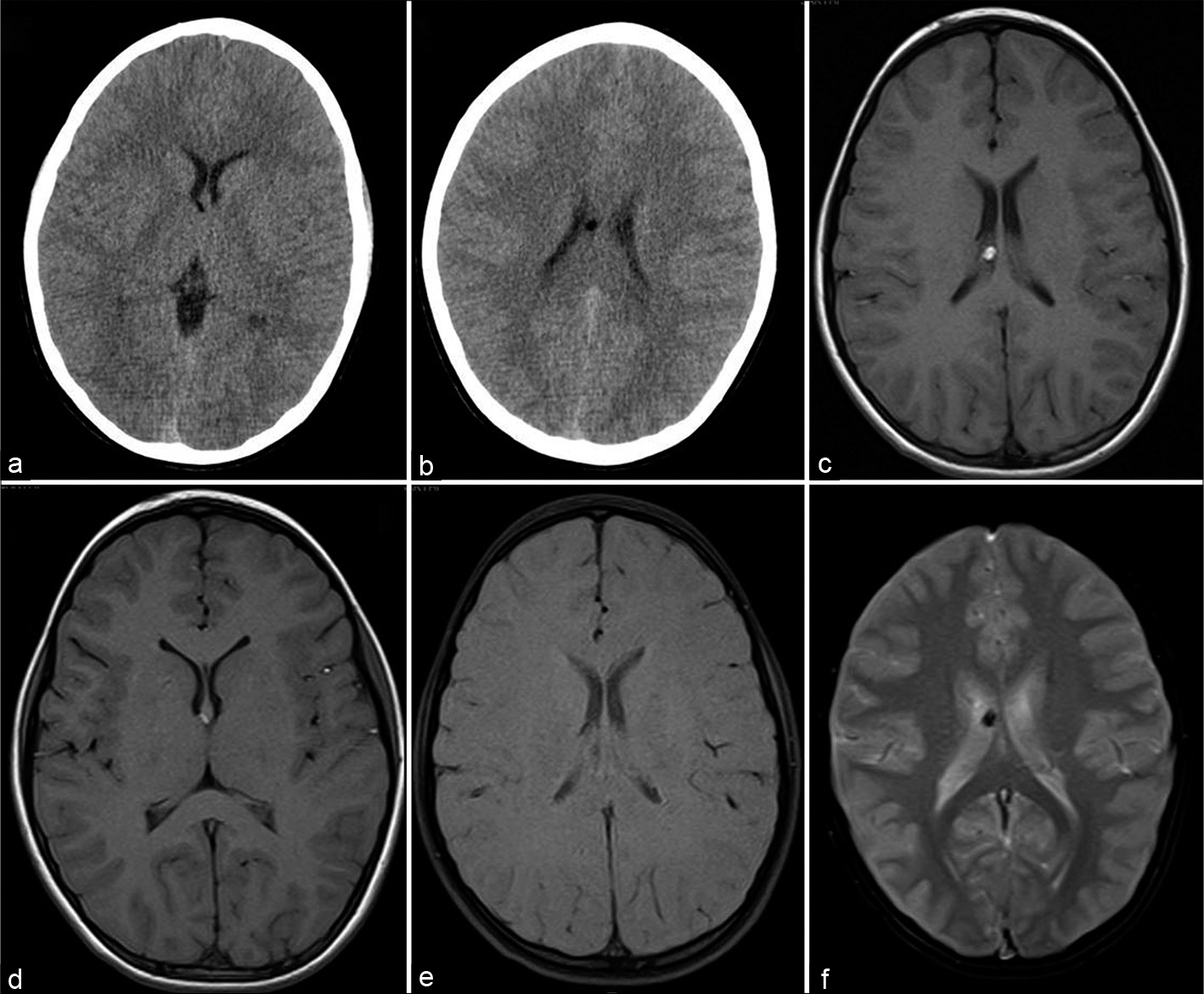
- A 12-year-old boy asymptomatic CT axial sections at ventricular level (a and b), T1-weigted axial (c and d), T1 with fat saturation (e), and gradient-echo GRE T2* axial (f) sections showing small fat specks along the right fornix with susceptibility effect.
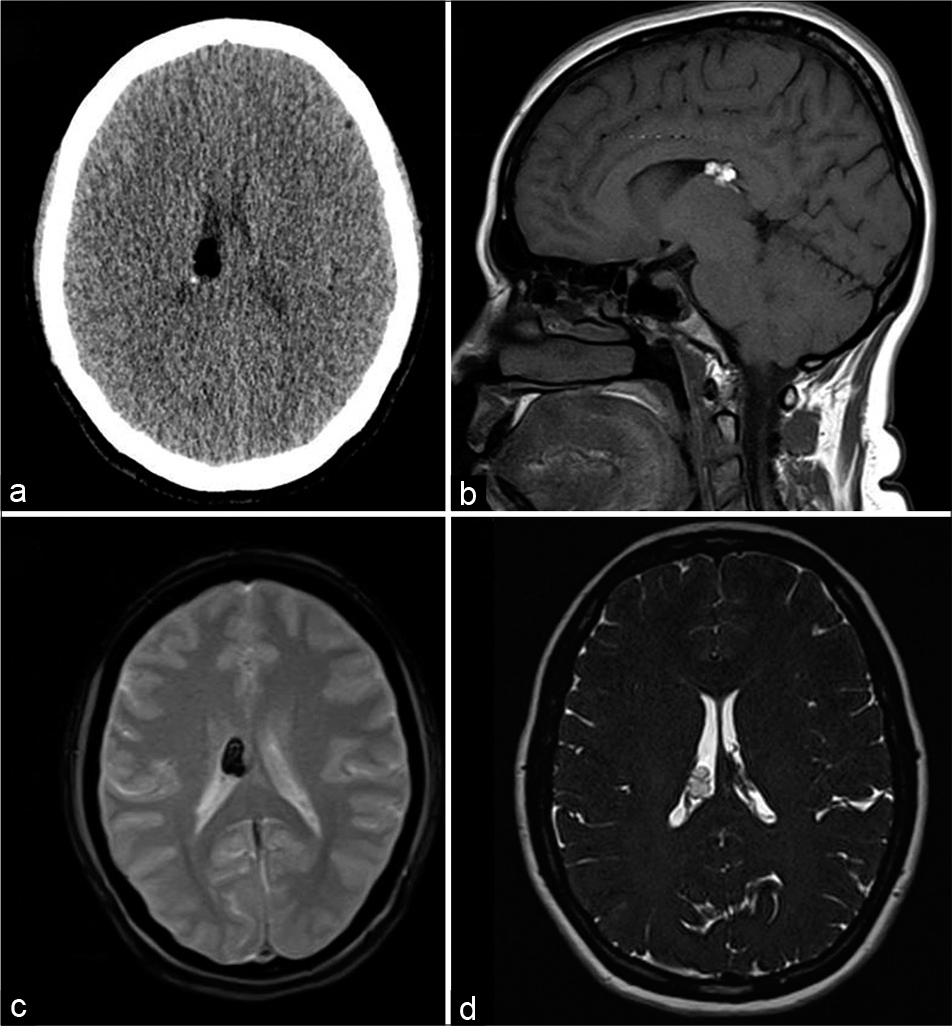
- A 39-year-old female with headache. Axial CT (a), T1-weighted sagittal (b), gradient-echo GRE T2* axial (c), and heavily T2-weighted SPACE axial (d) images showing lipoma along the choroid plexus of the right lateral ventricle with susceptibility effect (hypointensity in c).
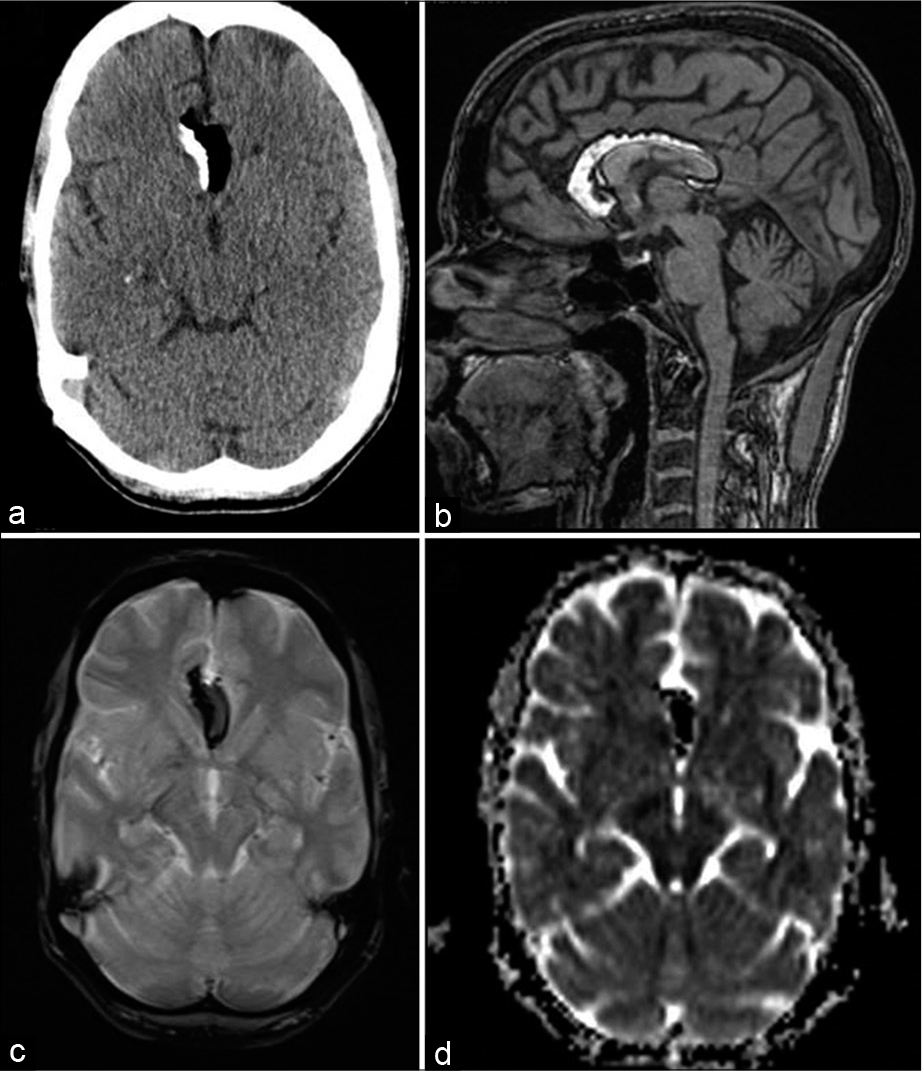
- A 45-year-old female with headache. Axial CT (a), T1-weighted sagittal, gradient-echo GRE T2* axial (c), and diffusion (ADC axial images showing partly-calcified pericallosal lipomas with susceptibility effects (c and d).
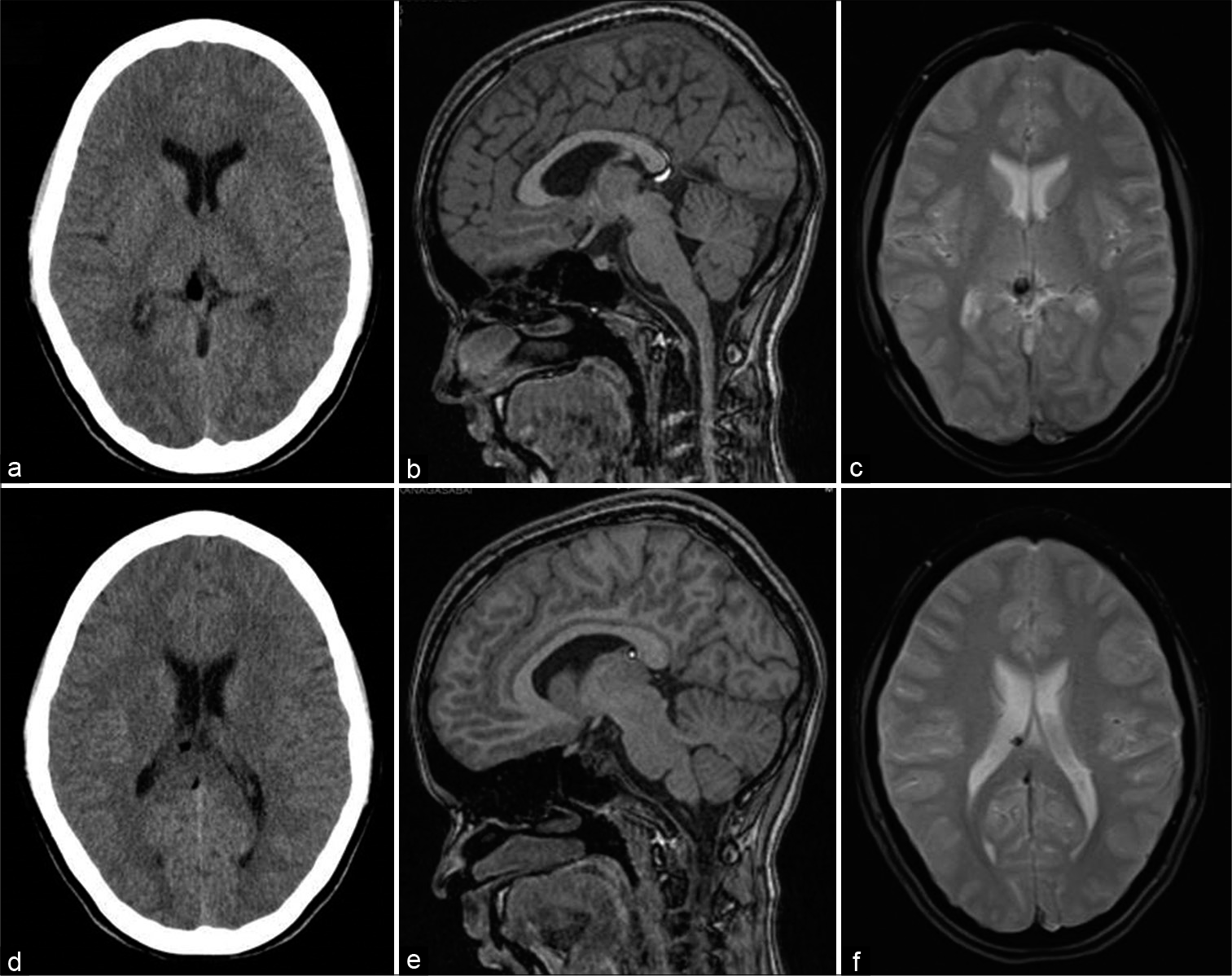
- A 13-year-old female with headache. Axial CT (a and b), T1-weighted sagittal (c and d), and gradient-echo GRE T2* axial (e and f) images showing small fat specks in the cistern of velum interpositum along the anterior end of the right fornix and just inferior to splenium of corpus callosum with susceptibility effects.
DISCUSSION
Intracranial lipomas are rare tumors constituting 0.1–1.3% of all cerebral tumors with reported autopsy incidence of 0.08–0.21%.[2] Initial reports of intracranial lipomas were mostly from the choroid plexus or pericallosal interhemispheric regions.[3] Bakshi et al.[7] in their study of 32 intracranial lipomas found eight in choroid plexus (25%), six along the convexity, five pericallosal, four quadrigeminal cistern, two each in suprasellar cistern, middle cranial fossa, retrocerebellar, along the tentorium, and one in the septum pellucidum. Yildiz et al.[8] reported 24 patients with intracranial lipomas – seven in quadrigeminal, four pericallosal, three each in sylvian, interhemispheric, and intercerebellar regions, two in choroid plexus, and one along body of fornix. Jabot et al.[9] found 45% of the lipomas interhemispheric, 25% quadrigeminal/superior cerebellar cistern, 14% along suprasellar/interpeduncular cistern, 9% CP angle cistern, and 5% sylvian cistern. Although the reported incidence in routine brain CT examinations (0.06–0.30%)[3] is less, Gossner[10] reported nine patients of intracranial lipomas from 50 CT scans including eight in midline locations and two around the brainstem. In their series, Lingegowda et al.[6] reported five pericallosal, four quadrigeminal, and one each in ambient cistern, parafalcine, and right parietal regions. Two of our cases showed multiple lesions. One patient had two lesions in the body of fornix and the other in the cistern of velum interpositum. This is similar to the previous report which suggest satellite and/or contiguous lesions may occur in intracranial lipomas.[3]
On CT, lipomas show fat density (from −40 to −100) and CT may also depict calcification, commonly in interhemispheric and suprasellar lipomas, which appear curvilinear around the periphery of the lesion or central and nodular. Lipomas in other locations usually do not show any calcification.[6] Among our cases, one pericallosal lipoma and another lipoma in lateral ventricle showed calcification. Morphologically, the pericallosal lipomas are further subclassified into tubulonodular and curvilinear types – tubulonodular lipomas are rounded or lobular lesions measuring more than 2 cm in thickness, and curvilinear pericallosal lipomas are posteriorly situated and appear elongated with thickness <1 cm.[11] Lipomas in other locations are of either curvilinear (length twice that of thickness) or nodular/oval types.
On MRI, lipomas show hyperintensity on T1-weighted and hyperintensity in fast spin-echo (FSE) T2-weighted MR images with complete suppression of signal on fat saturated sequences. Significant hypointensity due to susceptibility effect on SWI images has been described and is attributed to the microscopic calcium deposition within the lesion and chemical shift artifact.[6] Smith et al.[12] first observed this artifact and showed this effect increases with magnetic field strength and field-of-view (FOV). They mentioned that this effect may be reduced by increasing the bandwidth or asymmetric sampling to lower the time-to-echo (TE), but resulting in the lower signal-to-noise ratio SNR with the former. Bakshi et al.[7] found that lipomas showed hypointensity in conventional spin-echo (SE) T2/PD sequences, which was absent (and appeared hyperintense) with FSE sequences due to wider bandwidth and steeper gradients. They also pointed out that the T1 hyperintensity and chemical shift artifact may confuse with other lesions including hemorrhage. Peter et al.[5] described two types of chemical shift artifact in lipomas – the first along the frequency-encoding direction resulting in dark and hyperintense bands at fat-water interface and could be minimized by reducing the FOV, bandwidth increase, and lower field strength. The second effect is seen only in GRE sequences with fat and water in same voxel cancelling their opposite-phase transverse magnetization and may be corrected with appropriate TE (and field strength). The chemical GRE shift phenomenon occurs at lipid and water interface and is more prominently seen on gradient echo sequences. In our series, all seven cases showed this artifact on GRE images.
The main differential diagnosis for intracranial lipomas is the dermoid cysts. Dermoids are benign, congenital ectodermal inclusion cysts, commonly located in the midline, sellar/parasellar, vermis, and fourth ventricle regions. Both lipomas and dermoids show fat-density in CT and appear hyperintense on T1-weighted MRI, but the differences are: Dermoids can have non-fatty component like cartilage with or without calcifications, they can cause vessel displacement rather than incorporation by lipomas, and they do not appear to show this susceptibility or chemical shift effect.[3,8]
Although the combination of T1 hyperintensities and susceptibility effect may mimic subacute hemorrhage, the morphology and location of isolated or patchy foci in specific sites of subarachnoid spaces or the choroid plexus, in patients without any clinical presentation of hemorrhage currently or in the past, will help to identify the lipomatous nature of these lesions.
CONCLUSION
In this short case series, we report an interesting but less reported finding of susceptibility or chemical shift artifact intracranial lipomas which can potentially mislead with hemorrhage. The clinical importance is emphasized due to the incidental finding of these lipomas against the more serious nature of misinterpreting them as hemorrhagic component.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pathogenesis of intracranial lipoma: An MR study in 42 patients. AJR Am J Roentgenol. 1990;155:855-64.
- [CrossRef] [PubMed] [Google Scholar]
- Fat-water interface on susceptibility-weighted imaging and gradient-echo imaging: Comparison of phantoms to intracranial lipomas. AJR Am J Roentgenol. 2013;201:902-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mimics and diagnostic pitfalls of intracranial lesions in conventional MRI: Clues on advanced MRI. Neurol Asia. 2015;20:161-5.
- [Google Scholar]
- Susceptibility artifacts in lipomas. Neurol India. 2013;61:56-9.
- [CrossRef] [PubMed] [Google Scholar]
- MRI findings in 32 consecutive lipomas using conventional and advanced sequences. J Neuroimaging. 1999;9:134-40.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial lipomas: Importance of localization. Neuroradiology. 2006;48:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial lipomas: Clinical appearances on neuroimaging and clinical significance. J Neurol. 2009;256:851-5.
- [CrossRef] [PubMed] [Google Scholar]
- Small intracranial lipomas may be a frequent finding on computed tomography of the brain. A case series. Neuroradiol J. 2013;26:27-9.
- [CrossRef] [PubMed] [Google Scholar]
- Curvilinear and tubulonodular varieties of lipoma of the corpus callosum: An MR and CT study. J Comput Assist Tomogr. 1991;15:805-10.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial chemical-shift artifacts on MR images of the brain: Observations and relation to sampling bandwidth. AJR Am J Roentgenol. 1990;154:1275-83.
- [CrossRef] [PubMed] [Google Scholar]






