Translate this page into:
Superficial temporal artery-middle cerebral artery bypass signal intensity in time of flight magnetic resonance angiography as a marker of cerebral perfusion after revascularization procedure in moyamoya disease patients
*Corresponding author: Paramjeet Singh, Department of Radiodiagnosis, Post Graduate Institute of Medical Education and Research, Chandigarh, India. param.pgidr@yahoo.in
-
Received: ,
Accepted: ,
How to cite this article: Singh T, Singh P, Ahuja C, Aggarwal A, Gupta SK. Superficial temporal artery-middle cerebral artery bypass signal intensity in time of flight magnetic resonance angiography as a marker of cerebral perfusion after revascularization procedure in moyamoya disease patients. J Neurosci Rural Pract. 2024;15:587-93. doi: 10.25259/JNRP_162_2024
Abstract
The study was conducted to evaluate whether superficial temporal artery-middle cerebral artery (STA-MCA) bypass signal intensity has any correlation with cerebral perfusion on arterial spin labeling (ASL) perfusion in patients with Moyamoya disease (MMD), who underwent cerebral revascularization. Seventeen patients, who had STA-MCA bypass, were enrolled in this observational study and subjected to be followed up with MRI at three months post-operative interval. Region of interest (ROI) was placed in time of flight magnetic resonance angiography (TOF-MRA) images on STA-MCA bypass on the side where surgery was done. Signal intensity (SI) values obtained were normalized by converting them into ratio by taking signal intensity values of the common carotid artery just before its bifurcation as the denominator. The obtained STA-MCA bypass SI ratios were then compared with normalized cerebral blood flow (CBF) values obtained on ASL perfusion to look for correlation. Data analysis was done using SPSS software version 20.0 and GraphPad Prism 6. Spearman’s rank correlation coefficient was calculated between TOF-MRA and ASL MR perfusion parameters. A statistically significant positive correlation was found between STA-MCA bypass signal intensity with CBF of the frontal lobe (r = 0.532; P = 0.0280), CBF of the parietal lobe (r = 0.716; P = 0.001), and CBF of the temporal lobe (r=0.52; P = 0.033). However, there was no significant correlation between STA-signal intensity and CBF of basal ganglia (r = 0.128; P = 0.626).
Keywords
Brain
Cerebral revascularization
Moyamoya disease
Magnetic resonance angiography
Perfusion
INTRODUCTION
Moyamoya disease (MMD) is a cerebrovascular disorder, which affects the intracranial vessels, particularly those in the anterior circulation.[1,2] This disease causes progressive occlusion primarily of the supraclinoid segments of bilateral internal carotid arteries (ICAs), often involving the middle cerebral artery (MCA) and anterior cerebral arteries (ACAs). The etiopathogenesis of this disease is poorly understood. In 1998, initial criteria were established for the angiographic assessment and diagnosis of MMD. According to the criteria, the diagnosis depends upon stenosis of the supraclinoid segment of ICA’s and its extension into the proximal ACA and MCA. It is associated with collaterals in the basal cisterns, without any causal disease.[3,4] Surgical revascularization is suggested for patients having impaired hemodynamics and presenting with recurrent clinical symptoms due to decreased regional cerebral blood flow (CBF) and having vascular response and reserves in perfusion studies. Surgical anastomotic procedures include direct anastomoses (superficial temporal artery-MCA [STAMCA] anastomosis),[5] indirect procedures, and combination therapies. STA-MCA anastomosis is considered the standard surgical method to treat MMD. Post-operative evaluation after STA-MCA anastomosis is important to assess changes in cerebral perfusion and define the success of the procedure. Modalities such as positron emission tomography (PET)/single photon emission computed tomography (SPECT) perfusion imaging have been extensively used to study hemodynamics in these patients; however, these modalities have radiation-induced risks which are relevant in this population of young patients as multiple follow-up studies are usually required. The arterial spin labeling (ASL) perfusion technique is a good tool to study cerebral perfusion having no risk of radiation; however, this sequence is not available in most magnetic resonance imaging (MRI) centers. On the other hand, time-of-flight magnetic resonance angiography (TOF-MRA) is easily available in most magnetic resonance (MR) scanners and is easy to interpret.[6] The status of the STA-MCA bypass can also be easily examined through TOF-MRA as it gives good information regarding the status of vessels. We aim to evaluate whether the SI of STA-MCA bypass in TOF-MRA can predict perfusion status in post-operative patients.
MATERIALS AND METHODS
The study was approved by the Institute Review Board, and seventeen patients of MMD who had STA-MCA bypass were included over an 18-month duration (January 2019–June 2020). The subjects consisted of 11 male and 6 female patients, with ages ranging from 4 to 53 years (mean-19.6). The patients were explained about the risks and possible benefits of the procedure and written informed consent was obtained. Three months post-surgery, patients were followed up with imaging. The variables studied were STA-MCA bypass signal intensity (SI) and perfusion values on TOF-MR angiography (MRA) and ASL MR perfusion, respectively. TOF-MRA findings were then compared with ASL perfusion images to look for a correlation.
MRI was performed on a clinical 1.5-tesla MR system Siemens superconducting MR; (Magnetom Aera, Germany) using an 8-channel head neck-spine coil. No contrast injection was used. All the images were transferred to a Siemens Healthineers Workstation for data processing which involved the assessment of CBF parameters using color pictures of each selected lever. On ASL perfusion imaging, CBF maps were generated to obtain the CBF values of different regions of the brain [Figure 1]. The CBF values of the frontal, temporal, parietal lobe and basal ganglia were measured by putting the region of interest (ROI) on CBF map images. The CBF values obtained were normalized by converting them into ratios by taking the CBF values of the cerebellum as the denominator. The formula used for normalization was as follows: n CBF = CBF anastomosis side/CBF cerebellum. ROIs were placed on TOF-MRA source images on the STA-MCA bypass on the side where surgery was done [Figure 2]. SI values obtained were normalized by converting them into ratios by taking SI values of common carotid artery (CCA) just before its bifurcation as the denominator. The formula used for normalization was as follows: n STA-MCA bypass SI = STAMCA bypass SI operated side/CCA SI of ipsilateral side. The obtained STA-SI ratios were then compared with normalized CBF values to look for correlation. Data analysis was done using the Statistical Package for the Social Sciences software version 20.0 and GraphPad Prism 6. Spearman’s rank correlation coefficient was calculated between TOF-MRA and ASL MR perfusion parameters.
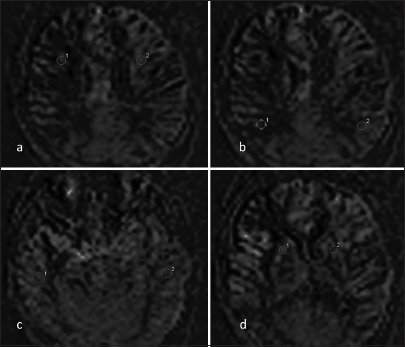
- Representative arterial spin labeling image of a patient who underwent right superficial temporal artery-middle cerebral artery bypass. Region of interests (1, 2) is shown in the bilateral symmetrical regions of the brain (a) Frontal lobe, (b) Parietal lobe, (c) Temporal lobe, and (d) Basal ganglia.
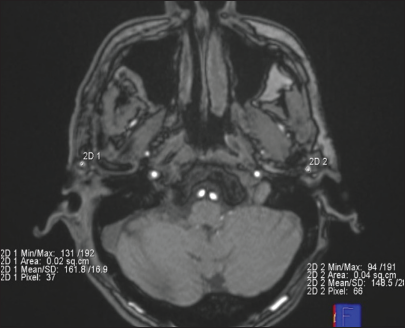
- An axial post-operative time-of-flight magnetic resonance angiography image of a patient who underwent right superficial temporal artery (STA)-middle cerebral artery anastomosis is shown. Bilateral regions of interest are manually placed on STA just proximal to its bifurcation. The mean signal intensity is shown in the bottom right and left of the image.
RESULTS
A total of 17 patients diagnosed with MMD were included in the study. The average age of the participants was 19.6 years, with a range of 4–53 years. The male-to-female ratio was 1.8:1. The main complaints of patients were seizures (23.5%), hemiparesis and weakness (17.7% each), and headache (11.8%). None of the patients had a family history of MMD. One patient had Down syndrome. 53% of patients underwent right STA-MCA bypass while the remaining 47% underwent left STA-MCA based on the involved hemisphere.
MRI findings
Out of 17 patients, 23.5% (4 patients) patients showed variable-sized and territory infarcts. Among these 75% of them had an infarct in the MCA territory and 25% (1 patient) had in ACA territory. Most of these infarcts were in the chronic stage. Infarcts <3 mm were considered small and more than 3 mm were considered large. In one patient, a large area of infarct was noted in the MCA territory and the rest had focal small infarcts. Encephalomalacia and gliosis changes were noted in 35.3% of the patients. The remaining 41% of patients showed no major cerebral parenchymal changes on MR images. The patency of STA-MCA was assessed on MRA and was determined based on visualization of the flow-related signal at the anastomotic site and downstream vessel. The graft vessels that were well visualized on the scan were considered to be patent and those which are not well visualized were considered to be non-patent. The SI of the bypass was measured by drawing the ROI of the size of the vessel. The STA-MCA bypass SI values were normalized. The quantitative analysis of CBF was done with ASL by applying ROI on bilaterally symmetrical areas and the values obtained were then normalized by taking CBF values of the cerebellum as a denominator to obtain the ratio. The normalized STA-MCA bypass SI values and normalized CBF values are shown in Table 1.
| Case no. | Normalized STAMCA bypass SI | Normalized frontal CBF | Normalized parietal CBF | Normalized temporal CBF | Normalized basalganglia CBF |
|---|---|---|---|---|---|
| 1 | 0.57 | 1.01 | 0.65 | 0.73 | 1.02 |
| 2 | 0.67 | 0.78 | 0.94 | 1 | 1.08 |
| 3 | 0.56 | 0.86 | 0.72 | 0.88 | 0.78 |
| 4 | 0.68 | 0.84 | 0.73 | 0.78 | 0.63 |
| 5 | 0.52 | 0.92 | 0.74 | 0.85 | 0.96 |
| 6 | 0.58 | 0.40 | 0.59 | 0.74 | 1.50 |
| 7 | 0.41 | 0.59 | 0.45 | 0.65 | 0.83 |
| 8 | 0.60 | 0.77 | 1.3 | 0.92 | 1.32 |
| 9 | 0.59 | 0.82 | 1.02 | 0.92 | 1.92 |
| 10 | 0.44 | 0.61 | 0.38 | 0.50 | 4.20 |
| 11 | 0.42 | 0.54 | 0.59 | 1.20 | 0.79 |
| 12 | 0.34 | 0.71 | 0.69 | 0.45 | 1.52 |
| 13 | 0.80 | 0.86 | 1 | 0.79 | 1.45 |
| 14 | 0.42 | 0.51 | 0.3 | 0.51 | 0.91 |
| 15 | 0.44 | 0.69 | 0.21 | 0.52 | 1.07 |
| 16 | 0.61 | 0.91 | 1.02 | 1.40 | 1.89 |
| 17 | 0.64 | 0.92 | 0.77 | 0.85 | 1.06 |
STAMCA: Superficial temporal arterymiddle cerebral artery, SI: Signal intensity, CBF: Cerebral blood flow
Correlation between STA-SI ratio and CBF
We evaluated the correlation between STA-MCA bypass SI with CBF in different lobes of the brain. Our study found a statistically significant positive correlation between STA-MCA bypass SI and CBF of the frontal lobe (r = 0.532; P = 0.0280), CBF of the parietal lobe (r = 0.716; P = 0.001), and CBF of the temporal lobe (r = 0.52; P = 0.033). However, there was no significant correlation between STA-MCA bypass SI and CBF of basal ganglia (r = 0.128; P = 0.626). The same has been shown in Figure 3.
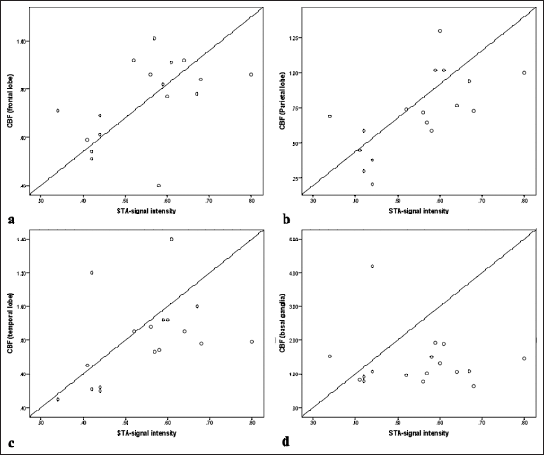
- X-axis represents superficial temporal artery-signal intensity (STA-SI) ratios: STA-SI ratio = STA-SI/CCA-SI, Y-axis represents normalized perfusion values of the (a) Frontal lobe, (b) Parietal lobe, (c) Temporal lobe, and (d) Basal ganglia. CBF: Cerebral blood flow, CCA-SI: Common carotid artery-signal intensity.
We further calculated the sensitivity of the STA-MCA bypass SI ratio in predicting cerebral perfusion of different regions of the brain, which was done by taking a standard value of normalized perfusion to be 0.8 in each lobe. All the perfusion values lesser than 0.8 were ranked/coded 0 and more than 0.8 were coded 1. Then, the receiver operating characteristic (ROC) curves were obtained for different lobes as shown in Figure 4. We found that the STA-MCA bypass SI ratio can predict the perfusion in frontal and parietal lobes with good sensitivity and specificity. The mean STA-MCA bypass SI ratio of 0.54 could predict good perfusion in the frontal lobe with a sensitivity of 87.5% and specificity of 67% and in the parietal lobe with a sensitivity of 83.3% and specificity of 55%.
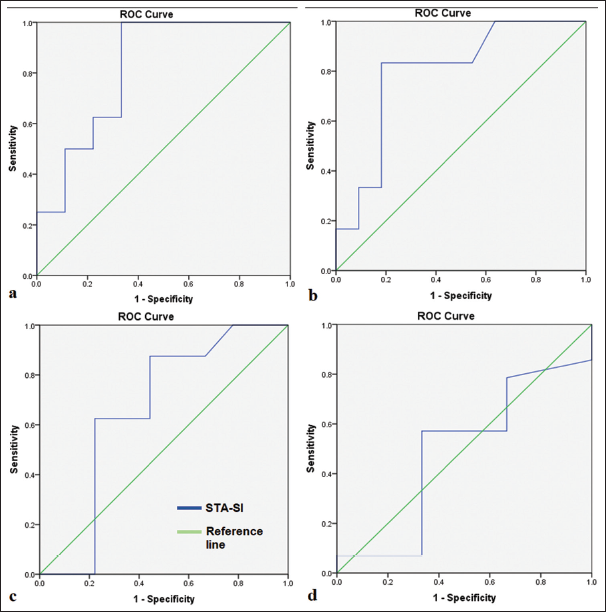
- Receiver operating characteristic (ROC) curve analysis for superficial temporal artery-signal intensity (STA-SI) and cerebral blood flow of different regions/lobes – (a) Frontal lobe, (b) Parietal lobe, (c) Temporal lobe, (d) Basal ganglia.
DISCUSSION
MMD is a cerebrovascular disease involving the intracranial vessels, mainly anterior circulation.[1,2] The disease primarily affects supraclinoid segments of bilateral ICAs and may extend to involve the proximal segments of the middle and ACAs. When ICA is completely blocked, it stimulates collateral formation from the posterior circulation to compensate for occluded intracranial anterior circulation flow.[7] Surgical revascularization has been recommended for patients who are symptomatic and have impaired cerebral hemodynamics. Surgical anastomotic procedures include direct anastomoses, indirect procedures, and combined therapies.[5,8] Direct anastomoses are considered as most effective method of treatment of MMD and are usually done between an STA and MCA. However, in MMD patients, intracranial artery hemodynamics is unstable and there is poor blood flow reserve; therefore, in direct anastomoses, cerebral hemodynamics can be altered, especially when the cerebral flow is suddenly restored. In some cases, bypass vessels become susceptible to occlusion, and it can lead to decreased perfusion and further compromise of the clinical condition due to hypoperfusion. Hence, it becomes very important to assess the perfusion status in these patients after surgery.[9]
Imaging modalities currently used for the same can be divided into two types: Quantitative and non-quantitative techniques. Quantitative modalities include studies such as PET, SPECT, perfusion computed tomography (CT), dynamic susceptibility contrast, ASL, and quantitative digital subtraction angiography (DSA) which are being used for cerebral hemodynamic changes. Non-quantitative modalities include different types of angiographies such as DSA, CT angiography, and MRA. Both types of modalities have their advantages and disadvantages.[10] Disadvantages include radiation exposure, invasiveness like in DSA, and issues of availability; hence, we need some simple non-invasive studies like MRI that are readily available. The studies by Ha et al. and Lee et al. have shown that ASL MRI can be a very useful tool in evaluating cerebral perfusion status in MMD patients.[11,12]
In our study, we applied the ROI on bilateral superficial temporal arteries (STAs) just before bifurcation, and mean SI values were obtained. Since SI values at these regions vary in individuals due to factors such as inhomogeneous magnetic fields, gradient differences, and patient properties, we decided to normalize these values with common carotid artery SI to obtain homogeneity across all subjects. In a study by Ha et al., they normalized the CBF values of different lobes of the brain by taking the CBF values of the cerebellum as the denominator to remove the transit time error of ASL. Similarly, we followed the same concept of normalization for perfusion on ASL.[12] In our study, we found that there was a statistically significant positive correlation between the STA-SI ratio of the STA-MCA bypass vessel and CBF (frontal lobe) (r = 0.532; P = 0.028), CBF (parietal lobe) (r = 0.716; P = 0.001), and CBF (temporal lobe) (r = 0.52; P = 0.033). However, there was no significant correlation between the STA-SI ratio and CBF (basal ganglia) (r = 0.128; P = 0.626). We further plotted ROC curves by taking the reference values of normalized perfusion to be 0.8 and checked how sensitive and specific the STA-SI ratio is in predicting cerebral perfusion. We found that if the STA-SI ratio is 0.54 or above, it indicates good perfusion in the frontal lobe (sensitivity 87.5% and specificity 67%) and parietal lobe (sensitivity 83% and specificity 55%). However, results were non-significant in predicting cerebral perfusion in the temporal lobe and basal ganglia. Similarly, in a study by Matsuo et al., they evaluated whether STA SI could be useful in predicting postoperative cerebral hemodynamics of patients with symptomatic ICA or MCA steno-occlusive disease. They correlated STA-SI ratios with STA duplex sonography and found a significant correlation of end-diastolic flow velocity ratio with STA-SI at a 1-year post-operative interval. This correlation might be useful to assess the bypass and its collateral bypass flow. They did not evaluate for cerebral perfusion changes. We further evaluated this concept by finding a correlation of STA-SI with perfusion values on ASL.[13] The results of the Sato et al. study also support the importance of TOF-MRA in evaluating cerebral perfusion changes in post-operative MMD patients.[14] A study by Ha et al. demonstrated that ASL MRI revealed a significant improvement in blood flow to the MCA territory following revascularization procedures in pediatric patients with MMD. The degree of this blood flow increase was closely correlated with the extent of collateral vessel formation as assessed by catheter angiography. Key studies highlighting the role of TOF-MRA and ASL perfusion in MMD patients are shown in Table 2.
| Authors | Year | Country | Scanner | Aim | Key result |
|---|---|---|---|---|---|
| Lee et al[11] | 2018 | Korea | 1.5 T or 3.0 T MR imagers (Signa HDxt, GE Healthcare, Milwaukee, Wis by using eight channel or 32 channel head coil | To determine whether arterial spin-labeling (ASL) magnetic resonance (MR) imaging could be used to identify changes in cerebral blood flow (CBF), collateral blood flow, and anastomosis site patency after revascularization in patients with moyamoya disease | ASL MR imaging can be used to identify perfusion changes in patients with moyamoya disease after revascularization as a noninvasive monitoring tool |
| Ha JY et al[12] | 2019 | Korea | 1.5T MRI system (MAGNETOM Avanto, Siemens Healthineers, Erlangen, Germany) with a 12-channel head coil | To determine the correlation between cerebral blood flow (CBF) on arterial spin labeling (ASL) MRI and the degree of postoperative revascularization assessed on digital subtraction angiography in children with moyamoya disease (MMD) | The nCBF values of the MCA territory obtained from ASL MRI increased after the revascularization procedure in children with MMD, and the degree of nCBF change showed a significant correlation with the degree of collateral formation evaluated via catheter angiography |
| Matsuo et al[13] | 2018 | Japan | "1.5 Tesla scanners (Signa Excite 1.5; GE Healthcare, Milwaukee, WI, Achieva 1.5 T; Philips HealthCare, Best, The Netherlands, and Magnetom Symphony 1.5 T; Siemens Medical Systems, Erlangen, Germany)" |
TOF MRA SI predicts the cerebral hemodynamic status after STA MCA anastomosis | STA-SI ratio may be useful to assess post-operative bypass flow. |
| Sato et al[14] | 2013 | Japan | 1.5-T imager (Signa Excite HD or HDxt or CV/I; GE Healthcare, Milwaukee, WI) | To evaluate whether 3D TOF MRA could be used as an alternative to SPECT for assessing the increase in regional cerebral blood flow (rCBF) after carotid endarterectomy | Significant correlation between the increase ratio of rCBF in ROI on SPECT with increase ration ofSI on MRA |
TOF-MRA: Time-of-flight magnetic resonance angiography, MMD: Moyamoya disease, ASL: Arterial spin labeling. STA-SI: Superficial temporal artery-signal intensity, MCA: Middle cerebral artery, TOF: Time of flight, ROI: Region of interest.
Limitations
The study is not without its limitations. The research was confined to a single institution and had a relatively small sample population. Second, the perfusion status with ASL has not been confirmed with DSA or SPECT, which is an established modality for the same and ASL perfusion values themselves can be misleading. Third, the position of ROI placement was subjective and may be associated with variations; however, choosing a standard site with a fixed area and normalization is likely to increase the consistency and reliability of the results. Fourth, STA-SI has been evaluated just proximal to its bifurcation which may not be entirely representative of the graft anastomotic site; however, this was the most suitable vessel site where ROI could best be placed.
CONCLUSION
Our study has shown that there is a statistically significant correlation between STA-SI ratios of STA-MCA bypass and ASL perfusion values of frontal, parietal, and temporal lobes of the brain; thus, STA-SI can be used for post-operative evaluation of cerebral hemodynamic changes in MMD patients. Moreover, additional conventional sequences in the same study can show that other brain parenchymal lesions associated with MMD and MRA can reveal abnormalities consistent with cerebral angiography.
Ethical approval
The study was approved by the Institutional Review Board at the Post Graduate Institute of Medical Education and Research, number INT/IEC/2019/001532, dated August 09, 2019.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Moyamoya disease: Case report and literature review. J Am Osteopath Assoc. 2009;109:547-53.
- [Google Scholar]
- MRI and MR angiography in moyamoya disease. J Magn Reson Imaging. 1998;8:762-6.
- [CrossRef] [PubMed] [Google Scholar]
- Combined treatment for Moya-Moya disease, by using direct anastomosis and revascularization: Experience of 225 operations. Zh Vopr Neirokhir Im N N Burdenko. 2007;3:11-6. discussion 16
- [Google Scholar]
- Novel magnetic resonance angiography stage grading for moyamoya disease. Cerebrovasc Dis Basel Switz. 2005;20:347-54.
- [CrossRef] [PubMed] [Google Scholar]
- New vessel formation in the central nervous system during tumor growth, vascular malformations, and Moyamoya. Curr Neurovasc Res. 2006;3:237-45.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management of moyamoya disease. Stroke. 2018;49:476-82.
- [CrossRef] [PubMed] [Google Scholar]
- Moyamoya disease in adults: The role of cerebral revascularization. Skull Base. 2005;15:27-41.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of Moyamoya disease and Moyamoya syndrome: Current status. J Comput Assist Tomogr. 2019;43:257-63.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring cerebral perfusion changes after revascularization in patients with moyamoya disease by using arterial spin-labeling MR imaging. Radiology. 2018;288:565-72.
- [CrossRef] [PubMed] [Google Scholar]
- Arterial spin labeling MRI for quantitative assessment of cerebral perfusion before and after cerebral revascularization in children with Moyamoya disease. Korean J Radiol. 2019;20:985-96.
- [CrossRef] [PubMed] [Google Scholar]
- Time-of-flight MRA signal intensity predicts the cerebral hemodynamic status after superficial temporal artery to middle cerebral artery anastomosis. J Clin Neurosci. 2019;59:124-9.
- [CrossRef] [PubMed] [Google Scholar]
- Signal intensity changes for the middle cerebral artery on 3-dimensional time-of-flight magnetic resonance angiography indicate acute hemodynamic changes after carotid endarterectomy. J Stroke Cerebrovasc Dis. 2013;22:e511-5.
- [CrossRef] [PubMed] [Google Scholar]






