Translate this page into:
Spontaneous Epidural Hematoma of the Cervical Spine Following Thrombolysis in a Patient with STEMI—Two Medical Specialties Facing a Rare Dilemma
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Spontaneous spinal epidural hematoma (SSEH) is a rare, albeit well-documented complication following thrombolysis treatment in ST elevation myocardial infarction (STEMI). A SSEH usually manifests with cervical pain and neurologic deficits and may require surgical intervention. In this case report, we present the first reported SSEH to occur following thrombolysis with reteplase. In this case, the SSEH manifested with cervical pain shortly after the patient emerged from his rescue percutaneous coronary intervention (PCI). Although magnetic resonance imaging reported spinal cord compression, the lack of neurologic symptoms prompted the treating clinicians to delay surgery. A dangerous dilemma emerged, as the usual antithrombotic regimen that was necessary to avoid stent thrombosis post-PCI, was also likely to exacerbate the bleeding. As a compromise, the patient only received aspirin as a single antiplatelet therapy. Ultimately, the patient responded well to conservative treatment, with the hematoma stabilizing a week later, without residual neurologic deficits. In conclusion, the conservative treatment of SSEH appears to be an acceptable option for carefully selected patients, but the risks of permanent neurologic deficits and stent thrombosis have to be weighted for each patient.
Keywords
spinal epidural hematoma
spontaneous spinal epidural hematoma
cervical spine
ST elevation myocardial infarction
thrombolysis
Introduction
A myocardial infarction with ST-segment elevation (STEMI) constitutes an emergency that confers significant morbidity and mortality to the patient, if left untreated. The gold standard therapy is primary percutaneous coronary intervention (PCI), preferably within the first 2 hours after the symptoms’ onset. If there is no access to a PCI center, thrombolysis with tissue plasminogen activators (tPA) is the treatment of choice.1
The benefits of thrombolysis outweigh the high risk of hemorrhage in most patients. Spontaneous spinal epidural hematoma (SSEH) is a rare and severe complication of the procedure.2 Several SSEH risk factors have been reported, such as hypertension, anticoagulant use, straining, lifting, and even sneezing, although it is unknown, whether these also predict SSEHs in STEMI patients. SSEH confers significant morbidity, as over 50% of patients are traditionally reported to retain at least some level of sensorimotor deficiency. Mortality remains high at 5.7%.3 In light of these figures, surgical decompression of the spine has been considered absolutely necessary, although a recent case series reported that 73% of patients treated conservatively presented complete resolution of neurological symptoms, implying that carefully selected patients can benefit from conservative treatment.4 Our case concerns a patient who fully recovered from an SSEH following thrombolytic therapy with reteplase, a recombinant tPA. This is the first time that reteplase is associated with this complication.
Case Report
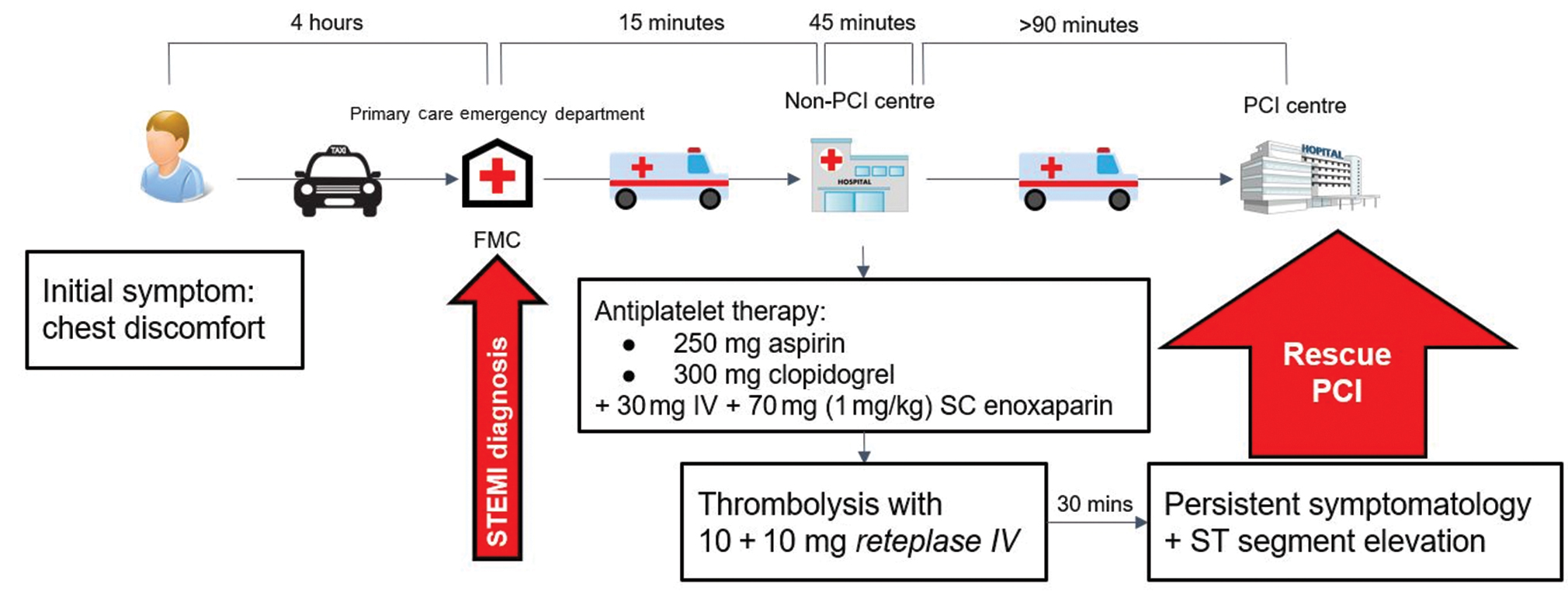
A 50-year-old Caucasian male was referred for medical consultation in a primary care emergency department, following chest discomfort of 4 hours onset. A diagnosis of an acute anterior wall STEMI was made and the patient was transferred immediately to the district General Hospital which was a non-PCI hospital. The nearest PCI-hospital was approximately 90 minutes away, so a decision for immediate thrombolysis was made, seeing as no absolute or relative contraindications to thrombolysis applied to the patient (Fig. 1).
-
Fig. 1 The patient’s timeline, from symptoms onset until the rescue PCI. FMC, First Medical Care; IV, intravenous; PCI, percutaneous coronary intervention; SC: subcutaneous.
Fig. 1 The patient’s timeline, from symptoms onset until the rescue PCI. FMC, First Medical Care; IV, intravenous; PCI, percutaneous coronary intervention; SC: subcutaneous.
The patient was admitted to the coronary care unit (CCU) and 250 mg of aspirin and 300 mg of clopidogrel were administered to him. A bolus of enoxaparin 30 mg was administered intravenously, followed by enoxaparin 70 mg (1 mg/kg, 100 mg max cumulative dose) subcutaneously. Thrombolysis was performed with a slow bolus of intravenous 10 U reteplase, followed by another 10 U of reteplase after 30 minutes. Another 30 minutes passed and the patient was still symptomatic with presence of ST-segment elevation, prompting the physician in charge to transfer the patient to the nearest PCI-hospital.
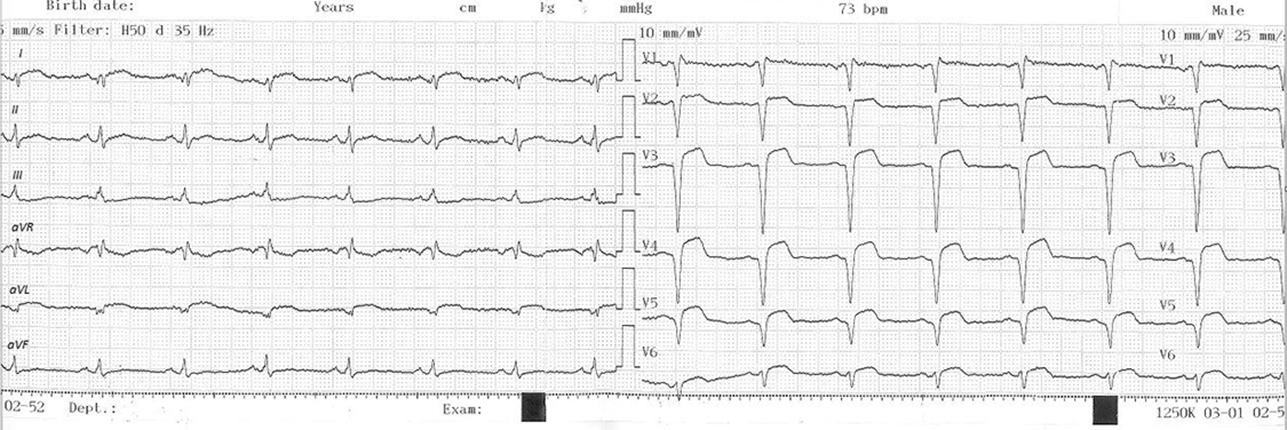
Upon arrival at the PCI hospital approximately 100 minutes later, the patient was hemodynamically stable, albeit still symptomatic, describing mild chest discomfort, mild shortness of breath, and moderate cervical pain (written down as headache at the time, in part due to the language barrier). Electrocardiogram (ECG) showed Q waves in V1–5 and ST-segment elevation of 2 mm in the same leads (Fig. 2). Cardiac enzymes were still elevated.
-
Fig. 2 12-lead ECG at the time of admission at the PCI-hospital, documenting recent anterolateral wall STEMI. ECG, Electrocardiogram; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
Fig. 2 12-lead ECG at the time of admission at the PCI-hospital, documenting recent anterolateral wall STEMI. ECG, Electrocardiogram; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
The patient was immediately transferred to the cardiac catheterization laboratory, where a dosage of 180 mg of ticagrelor was administered. Coronary angiography was performed through radial access.1 A critical mid left anterior descending (LAD) lesion was documented following coronary angiography and a drug-eluting stent (DES) was implanted successfully. No further heparin was used. Following angioplasty, the patient was transferred to the cardiac care unit, where he described consistent cervical headache that was first managed with opioids.
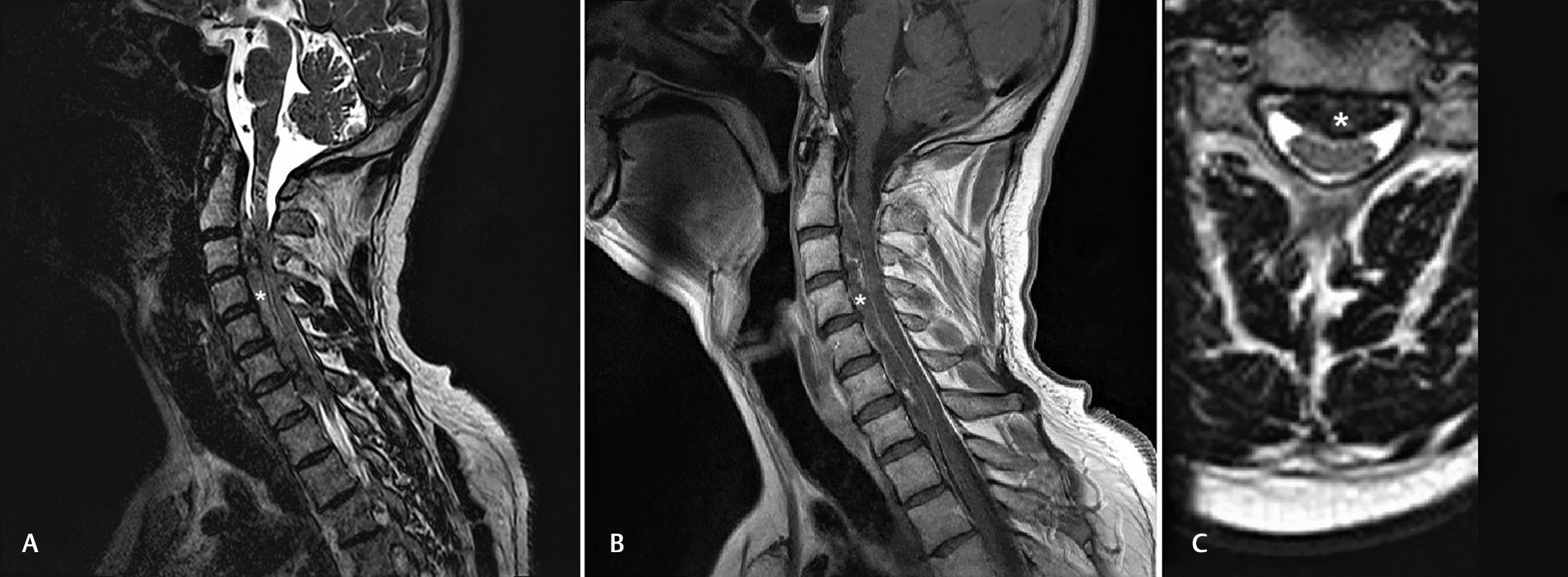
The patient had an uneventful night stay at the cardiac care unit, showing stable hemodynamics and complete resolution of dyspnea and chest discomfort. He was on double antiplatelet regimen with aspirin 100 mg and ticagrelor 90 mg bid in addition to pantoprazole 20 mg od, bisoprolol 5 mg od, atorvastatin 40 mg od, and furosemide 20 mg od. Echocardiogram revealed a global left ventricular ejection fraction of 45%, anterior mid and apical wall hypokinesis, and mild functional mitral valve regurgitation. The single most significant symptom was still the cervical pain that steadily intensified and showed significant resistance to various analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs). The patient was transferred to the Cardiology ward and was further referred for orthopaedic and neurosurgery consultation. Clinical examination showed no neurologic signs, but a cervical magnetic resonance imaging (MRI) was performed that revealed an extended C2–T2 cervical epidural hematoma (Fig. 3).
-
Fig. 3
MRI images at the time of diagnosis. (A) Sagittal T2-weighted MRI image. The intensity of the hematoma is lower than normal tissue on this MRI sequence. The extension of the hematoma is from C2 vertebra to T2 vertebra. (B) Sagittal T1-weighted MRI image. (C) Axial T2-weighted MRI image. The hematoma’s intensity is lower than normal tissue. (The hematoma is indicated with a white asterisk) MRI, magnetic resonance imaging.
Fig. 3 MRI images at the time of diagnosis. (A) Sagittal T2-weighted MRI image. The intensity of the hematoma is lower than normal tissue on this MRI sequence. The extension of the hematoma is from C2 vertebra to T2 vertebra. (B) Sagittal T1-weighted MRI image. (C) Axial T2-weighted MRI image. The hematoma’s intensity is lower than normal tissue. (The hematoma is indicated with a white asterisk) MRI, magnetic resonance imaging.
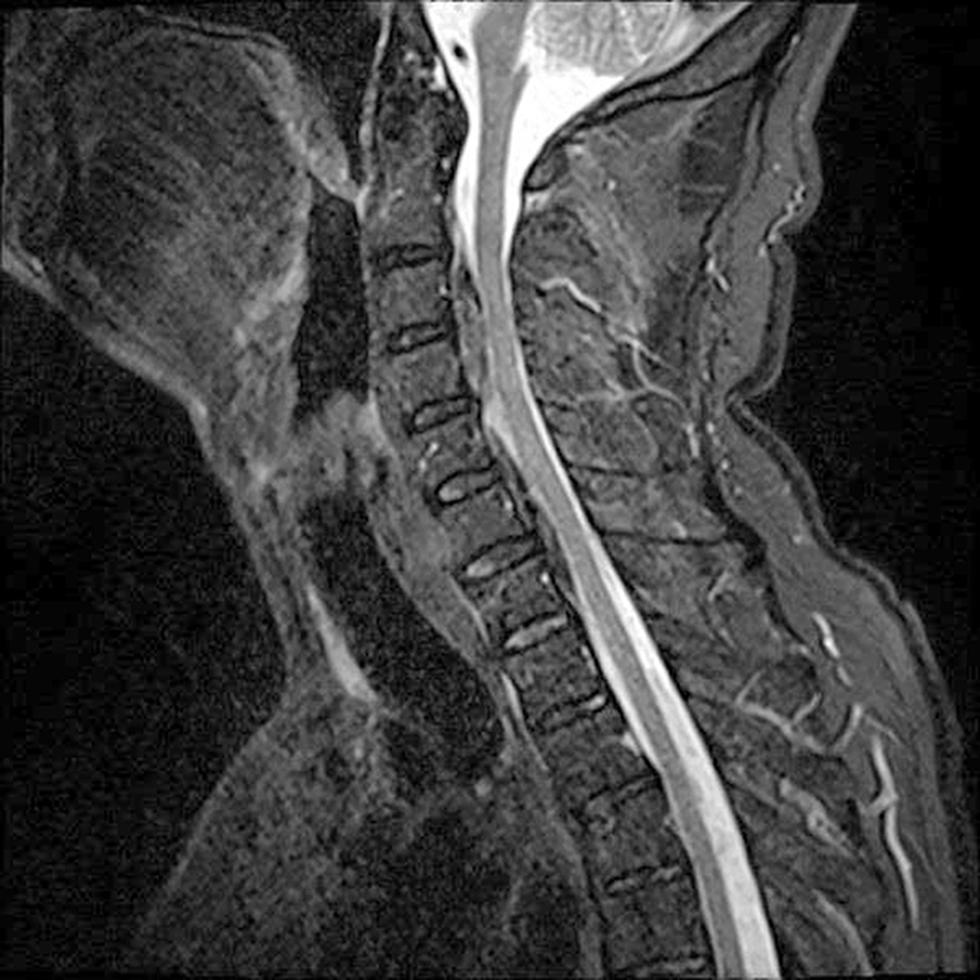
Following the diagnosis of the hematoma, ticagrelor was discontinued and aspirin 100 mg remained as the only antiplatelet drug. A mixture of various analgesics and NSAIDs was used to manage the patient’s symptoms that showed significant improvement in 72 hours, following ticagrelor discontinuation. A second MRI, obtained 7 days later, confirmed the stabilization and slight diminution of the hematoma. Throughout all this time, the troponin levels remained low, indicating stent patency (Fig. 4).
-
Fig. 4 Midsagittal T2-weighted MRI indicating the regression of the hematoma one week after the initial occurrence. MRI, magnetic resonance imaging.
Fig. 4 Midsagittal T2-weighted MRI indicating the regression of the hematoma one week after the initial occurrence. MRI, magnetic resonance imaging.
The patient was visiting Greece for vacation and was repatriated 10 days later with the consultation of performing a novel MRI exam on the following days. Since the third MRI imaging showed further diminution of the hematoma, clopidogrel was initiated. The authors’ last contact with the patient was 10 months post-STEMI. The patient was healthy and on double antiplatelet therapy, with no clinically discernible sequelae from the hematoma. Clopidogrel was scheduled to be discontinued 12 months following PCI.
Discussion
To the extent of the authors’ knowledge, this is the first case described in the literature of spinal epidural hematoma associated with reteplase use. But was the spinal epidural bleeding brought about by the administration of reteplase? This question might arise while examining this case and could also bear significant medicolegal implications.
Before reteplase is causally linked to the hematoma, it should be stated that SSEH has also been reported in patients treated for Non-STEMI,5 who receive similar anticoagulation therapy without thrombolysis.6 Thus, a more likely explanation is that the dual antiplatelet therapy, combined with enoxaparin, thrombolysis, and ticagrelor loading, synergistically increased the probability of bleeding complications. In this case, in particular, the administration of a loading dose of ticagrelor so soon after the administration of 300 mg clopidogrel may have played a major role in the evolution of the hemorrhage.
That said, it is difficult to conclusively claim that this decision was incorrect, as there seems to be a gray area concerning the optimal antiplatelet therapy in the first 48 following STEMI, in patients who undergo rescue PCI. This switch is necessary, as ticagrelor demonstrably reduces mortality in STEMI patients compared with clopidogrel, without increasing the risk of postthrombolysis bleeding.7 8 Unfortunately, the guidelines don’t provide an optimal time to switch from clopidogrel to ticagrelor. Thus, the decision stands upon the discretion of the interventionalist in charge. A more conservative strategy in early switching of P2Y12 inhibitors following thrombolysis may have been a safer approach, postponing the switch to the post-PCI period.
The patient received a recommendation to resume dual antiplatelet therapy (DAPT) for 12 months post-PCI and then discontinue clopidogrel. The European Society of Cardiology (ESC) guidelines propose that DAPT should be continued during past 1-year post-PCI in high-risk patients, but it states that the risks and benefits of extending DAPT duration should be individually weighed, as prolonged DAPT appears to reduce cardiovascular deaths, while significantly increasing the risk of major bleeding.9
As regard to the clinical entity itself, SSEH is an emergency, and it is rapidly presented with severe back pain and signs of compression of the spinal cord/neurologic deficits requiring immediate surgical treatment.2 5 10 It may also be presented with breathing distress and paraplegia.11 Furthermore, it has been stated that even the initial symptoms of Guillain–Barre syndrome can be similar to a case of SSEH located at the cervicothoracic junction.12
The occurrence of SSEH has been associated with several conditions, such as hemodialysis, thrombolysis, or antiplatelet–anticoagulant therapy including aspirin, dabigatran, and rivaroxaban.13 14 15 16 MRI appears to be the gold standard imaging modality for diagnosis. Surgery and, more specifically, urgent decompressive laminectomy and evacuation of hematoma is indicated in several cases; however, it can occasionally be deferred if the patient demonstrates significant and rapid improvement.11 17 In general terms, the gold standard treatment choice still remains controversial.
In a recent study, Yu et al reviewed retrospectively 55 cases of SSHE, and they concluded that spinal epidural (extradural) arteriovenous fistula is a significant cause of SSEH indicating the need for spinal digital subtraction angiography, and they also recommended microsurgery as the preferred treatment strategy, while conservative treatment could not prevent occurrence of multiple episodes or rebleeding. Additionally, they identified risk factors such as poor clinical outcome for the advanced age at initial onset, short–progression interval, no symptom relief after admission, hypesthesia, complete spinal cord injury, and hematoma below the T4 level.18
On the other hand, Nagata et al retrospectively analyzed the clinical records of 10 patients with SSEH treated conservatively. They found that, in all patients, the neurological status improved within 24 hours and they concluded that the SSEH in the cervical spine required approximately 10 days for absorption.19 Raasck et al evaluated 65 cases of SSEH to compare the outcomes of surgical versus conservative treatment. They showed that conservative treatment is an effective alternative with good results and, in some cases, better recovery rates compared with the surgically managed patients.4 Hibi et al also reported successful recovery after conservative treatment in a patient with SSEH following hemodialysis.13
Although one case where the resolution of an SSEH in STEMI patient was achieved conservatively shouldn’t change a clinician’s approach to this clinical entity, it serves as a proof of concept that conservative management of such cases can help MI patients to avoid major surgery. As mentioned before, there is recent evidence that conservative treatment in the general SSEH population can be beneficial in selected patients.4 The degree of neurologic deficit and the time of improvement seem to be crucial factors for conservative treatment selection.4 13
In our case, the fear of a hematoma recurrence led to the clinician recommending only the minimum of DAPT. There is no current consensus on how such a major bleeding event should influence short- and long-term antithrombotic strategy, so such a clinical judgment is required.
STEMI patients that develop SSEH are an exceptionally rare subgroup of this population and very little is known about which findings apply to them. In these patients, there are no clear answers. Decompressing the hematoma is an appealing course of action that solves the dilemma and theoretically protects the patient from further neurologic deterioration. On the other hand, conservative management spares the patient the potential complications of major surgery in the immediate post-MI period, but limits the medications the physicians can use to preclude stent thrombosis.
Conclusion
It is true that spinal cervical epidural hematoma is a rare and tough to manage complication after thrombolysis. As a result, in the apparent lack of concrete and evidence-based recommendations, cardiologists and neurosurgeons should aim at a balanced cooperation and careful case management by estimating the risks and the benefits of every single case separately and by evaluating the very specific factors of each patient.
Conflict of Interest
None declared.
References
- ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119-177.
- [Google Scholar]
- Spinal epidural hematoma - A rare and debilitating complication of thrombolytic therapy. J Cardiovasc Dis Res. 2013;4(4):236-238.
- [Google Scholar]
- Experience in the surgical management of spontaneous spinal epidural hematoma. J Neurosurg. 2004;100(1):38-45. Suppl Spine
- [Google Scholar]
- Spontaneous spinal epidural hematoma management: a case series and literature review. Spinal Cord Ser Cases. 2017;3:16043.
- [Google Scholar]
- Spontaneous spinal epidural hematoma developing after percutaneous coronary intervention: early diagnosis, early intervention, and good outcome. Turk Kardiyol Dern Ars. 2016;44(2):158-160.
- [Google Scholar]
- ESC Scientific Document Group. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(3):267-315.
- [Google Scholar]
- Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057.
- [Google Scholar]
- Ticagrelor vs clopidogrel after fibrinolytic therapy in patients with st-elevation myocardial infarction a randomized clinical trial. JAMA Cardiol. 2018;3(5):391-399.
- [Google Scholar]
- Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791-1800.
- [Google Scholar]
- Spontaneous epidural hematoma of cervical spine. Int J Spine Surg. 2018;12(1):26-29.
- [Google Scholar]
- acute cervical and thoracic ventral side spontaneous spinal epidural hematoma causing high paraplegia: a case report. Anesth Pain Med. 2017;7(6):e14041.
- [Google Scholar]
- Spontaneous spinal epidural hematoma mimicking Guillain-Barre syndrome. Brain Dev. 2019;41(4):392-395.
- [Google Scholar]
- Successful recovery from spontaneous spinal epidural hematoma in a patient undergoing hemodialysis. Am J Case Rep. 2017;18:1357-1364.
- [Google Scholar]
- Spontaneous spinal epidural hematoma associated with the use of low-dose aspirin in elderly patient. J Am Acad Orthop Surg Glob Res Rev. 2018;2(10):e059.
- [Google Scholar]
- Spontaneous cervical spinal epidural hematoma associated with dabigatran. World Neurosurg. 2018;112:264-266.
- [Google Scholar]
- Spontaneous spinal epidural hematoma from rivaroxaban. Clin Pract Cases Emerg Med. 2018;2(2):151-154.
- [Google Scholar]
- Rapid recovery of spontaneous spinal epidural hematoma without surgical treatment: case report and literature review. World Neurosurg. 2018;115:216-219.
- [Google Scholar]
- Spontaneous spinal epidural hematoma: a study of 55 cases focused on the etiology and treatment strategy. World Neurosurg. 2017;98:546-554.
- [Google Scholar]
- Consecutive images of conservatively treated cervical spontaneous spinal epidural hematoma. J Clin Neurosci. 2019;59:270-275.
- [Google Scholar]






