Translate this page into:
Spontaneous Downbeat Nystagmus in Anti-GAD-Antibody-Associated Paraneoplastic Syndrome
Saraswati Nashi, DM National Institute of Mental Health and Neurosciences Hosur Road, Bengaluru -560029, Karnataka India nandanashi@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Spontaneous downbeat nystagmus and ocular flutter are rare clinical signs. Such findings are commonly related to cerebellar pathology, predominantly ischemia. In a significant percentage of patients, the cause may not be found. If these signs are associated with ataxia, cognitive decline, and seizure, anti-glutamic acid decarboxylase-associated neurological syndrome must be suspected. Background history of tumor has to be enquired. Treatment with immune modulation helps in partial recovery of such cases.
Keywords
anti-GAD antibodies
downbeat nystagmus
Introduction
Anti-glutamic acid decarboxylase (anti-GAD)-antibody-associated neurological syndromes include stiff-person syndrome, limbic encephalitis, epilepsy, cerebellar ataxia, and, rarely, myasthenia gravis.1 Downbeat nystagmus (DBN) is spontaneous nystagmus occurring with fixation in primary position or lateral gaze. Ocular flutter is a horizontal saccadic intrusion without intersaccadic interval. We describe a rare case of a middle-aged lady who presented with cerebellar ataxia, cognitive impairment, ocular flutter, and DBN with a background history of breast carcinoma on treatment.
Case Report
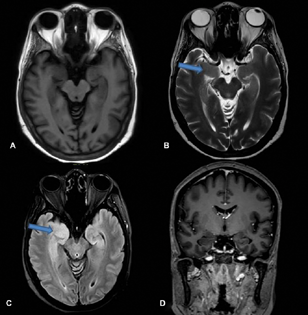
A 50-year-old female, teacher, right-handed, presented with complaints of imbalance while walking and slowness of activities of daily living for 11 months, blurred vision for 11 months, decreased interest in daily activities and forgetfulness for 10 months, and fearfulness and seizures for 6 months. She was asymptomatic till 1 year back when she started complaining that she gets easily fatigued while teaching in the class and had to take rest more often. While walking she developed a sense of imbalance and fear of falling, and she needed the support of a table or wall. For the last 4 months, she started using a stick while walking. She also developed difficulty in getting up from sitting position along with slowness of all other activities like bathing and brushing. For the last 9 months, she developed visual symptoms in that she was not able to see the students on the last bench; gradually she developed difficulty in reading the newspaper, or letters on the blackboard, and she completely stopped reading for the last 7 months. For the last 7 months, she remained confined to a room and stopped interacting with family members. Around 10 months back she developed forgetfulness in the form that she forgot her daughter's and grandson's birthday. She started forgetting the dates of the various bills to be paid, forgetting conversations and forgetting what she had for meals. For the last 5 to 6 months she started having episodes of fearfulness, tonic posturing of both upper and lower limbs, clenching of teeth, and tongue bite followed by loss of consciousness for 3 to 5 minutes, suggestive of complex partial seizures with secondary generalization. The electroencephalogram was normal. Frequency of such episodes was once in a week. There was no history of myoclonus, autonomic symptoms, movements during sleep, or weight loss. Four months earlier, she was diagnosed to have type II diabetes mellitus. The patient is a known case of carcinoma of left breast and underwent total excision of the lesion 6 years earlier followed by radiotherapy. She was started on trastuzumab. General physical and systemic examination was normal. Assessment of higher mental functions revealed impaired attention, memory, and language. Cranial nerve examination showed DBN and ocular flutter (Video 1), while rest of the cranial nerves were normal. Power and sensations were normal. Deep tendon reflexes were sluggish (grade 1). She had normal upper limb coordination but ataxic gait. Her fasting and postprandial blood glucose was 324 mg/dL and 498 mg/dL, respectively. The HbA1c level was 12%. Erythrocyte sedimentation rate was 10 mm in first hour. Serum folate levels (20.0 ng/mL), B12 (960 pg/mL), and thyroid stimulating hormone levels (2.6 mU/L) were normal. Insulin levels were not assessed. Serum anti-GAD-65 titer was high: >280 (0–17 IU/mL). Magnetic resonance imaging (MRI) of the brain (Fig. 1) revealed T2 fluid-attenuated inversion recovery hyper-intensity in bilateral medial temporal lobes (Fig. 1).
-
Fig. 1 Magnetic resonance imaging showing: normal T1 (A); hyper-intensities in bilateral medial temporal areas in T2 (B) and fluid-attenuated inversion recovery (C); and no enhancement in T1 contrast image (D).
Fig. 1 Magnetic resonance imaging showing: normal T1 (A); hyper-intensities in bilateral medial temporal areas in T2 (B) and fluid-attenuated inversion recovery (C); and no enhancement in T1 contrast image (D).
The patient was treated with intravenous steroids (5 g in a month) and plasma exchange (PE; five cycles of large volume of PE every month) for 4 months. Ataxia improved significantly. Cognition, ocular flutter, and DBN remained the same.
Discussion
We report a middle-aged lady who presented with complaints of progressive imbalance while walking, slowness of activities of daily living, blurred vision, decreased interest in daily activities, forgetfulness, fearfulness, and seizures. Symptoms evolved over 11 months. Examination showed cognitive impairment, DBN, ocular flutter, and ataxia. She tested positive for anti-GAD antibodies and had past history of breast carcinoma. We describe the clinical course, clues for the diagnosis, treatment, and outcome in our patient with a brief review of literature.
A combination of ataxia, cognitive decline, and DBN is a clue for anti-GAD-associated syndrome.2 3 In a series of 62 patients4 with anti-GAD paraneoplastic autoantibody evaluation, neurological manifestations were multifocal in 41 (66%) patients and included cerebellar ataxia (63%), brainstem involvement (29%), seizures (27%), stiff-man phenomenon (26%), extrapyramidal signs (16%), and myelopathy (8%). One-third of the patients had type 1 diabetes.4 This condition is associated with other autoimmune polyendocrinopathies like thyroid dysfunction and type 1 diabetes mellitus.1 In our patient, ataxia was the first symptom, which is the most common presentation. Later she developed cognitive decline and eye movement abnormalities.
DBN is defined as spontaneous nystagmus present with fixation in the primary position or on lateral gaze, characterized by downward fast phase. A proportion of patients with DBN have an identifiable anatomical lesion of the vestibulocerebellum and underlying medulla. The cerebellar flocculus inhibits anterior canal pathways. Consequently, experimental lesions to the vestibulocerebellum (flocculectomy in the monkey) remove inhibition from the anterior, though not posterior, canal projections and produce DBN. The GABAergic transmission from Purkinje cells to floccular neurons has been suggested to be involved. It is possible that a primary action of GAD-Ab against GABAergic neurons induces chemical denervation of the floccular neurons, thereby causing DBN.
In a study done on 117 patients with DBN, in 62% of patients the etiology was identified (“secondary DBN”), the most frequent causes being cerebellar degeneration and cerebellar ischemia.5 In 38%, no cause was found (“idiopathic DBN”). A major finding was the high co-morbidity of both idiopathic and secondary DBNs with bilateral vestibulopathy (36%) and the association with polyneuropathy and cerebellar ataxia even without cerebellar pathology on MRI. Our patient had DBN and ocular flutter, which were persistent even after treatment.
DBN and ocular flutter are associated with an anti-GAD-associated syndrome but rarely reported. Few case reports are published in the literature. To our knowledge, this is the first report from India. Isolated presence of DBN and ocular flutter localizes to brain stem pathology. Association with ataxia, cognitive decline, and seizures should hint us a possibility of paraneoplastic syndrome.
The treatment of paraneoplastic syndrome has to be aimed at localizing the primary and treating it first. There are no randomized studies on treatment of GAD-antibody-associated syndrome. Neurologic syndrome is primarily due to immunologic mechanism. Hence, steroids, intravenous immune globulins, PE, and other immune suppressants like rituximab are tried. Few studies reported better improvement with PE than intravenous steroids. Our patient was treated with PE and intravenous steroids and motor symptoms were controlled, but cognition and abnormal eye movements remained the same.6
Conclusion
Ocular flutter and spontaneous DBN are rare features in anti-GAD-associated paraneoplastic syndrome. Any patient with such abnormal eye movements, cognitive decline, and ataxia should be further investigated for paraneoplastic antibodies and treated accordingly.
Conflict of Interest
None declared.
Funding None.
References
- Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553-2563. (Pt 10):
- [Google Scholar]
- Spontaneous downbeat nystagmus as a clue for the diagnosis of ataxia associated with anti-GAD antibodies. J Neurol Sci. 2015;359:21-23. (1-2):
- [Google Scholar]
- Autoantibodies to glutamic acid decarboxylase in downbeat nystagmus. J Neurol Neurosurg Psychiatry. 2003;74(7):998-999.
- [Google Scholar]
- Glutamic acid decarboxylase autoimmunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clin Proc. 2006;81(9):1207-1214.
- [Google Scholar]
- Downbeat nystagmus: aetiology and comorbidity in 117 patients. J Neurol Neurosurg Psychiatry. 2008;79(6):672-677.
- [Google Scholar]
- Limbic encephalitis with antibodies to glutamic acid decarboxylase presenting with brainstem symptoms. Ann Indian Acad Neurol. 2015;18(2):243-245.
- [Google Scholar]







