Translate this page into:
Serum Interleukin-6 is Not Linked with Sleep-Quality, Restless Legs Syndrome, and Depression, But with 6-Month Survival in Hematological Malignancies
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Serum interleukin (IL)-6 has been found to be associated with sleep quality, mood, and survival in patients with solid tumors. Results in these studies were confounded by knowledge of diagnosis to study subjects. Moreover, such data among subjects with hematological malignancies and data regarding restless legs syndrome is limited. The present study was, therefore, conducted to assess the sleep quality, depression, and restless leg syndrome in hematological malignancies and to study if there is any role of IL6 associated with it.
Methods:
Sixty-six subjects having hematological malignancy were included in this study after excluding the potential confounders. Sleep quality was examined using Pittsburg Sleep Quality Index, depression by the Patient Health Questionnaire-9. Diagnosis of RLS was made through clinical examination. Serum for measurement of IL-6 was collected at baseline and after 1 month of initiation of chemotherapy. Patients were followed up for 6 months.
Results:
Average age of study subjects was 50.16 years with male predominance. Nearly 22.7% had clinical depression, 28.8% had poor quality sleep, and restless legs syndrome (RLS) was reported in 6.1% cases. Nearly 22.7% patients died at 6 months. Disturbed sleep at baseline was associated with depression (odds ratio [OR] =7.89) and poor 6 months survival. Serum IL-6 did not show any association with sleep quality, restless-legs-syndrome, and depression. However, baseline high level of serum IL-6 (OR = 26.06) and low level after chemotherapy (OR = 0.03) were associated with poor survival at 6 months.
Conclusion:
Poor quality sleep, depression, and RLS are prevalent among adult subjects with hematological malignancies. Sleep disturbance, high pretreatment inflammatory and lowering of inflammatory load after chemotherapy increase likelihood for poor prognosis. Serum IL-6 did not show any association with sleep quality, restless legs syndrome and depression.
Keywords
Hematological malignancy
interleukin-6
inflammation
prognosis
restless leg syndrome
sleep
INTRODUCTION
Sleep disturbances are common among patients with active malignancy and also among cancer survivors, affecting nearly one-third to half of these patients.[1234] Pain, emotional distress, and chemotherapy predict sleep disturbances; however, site of cancer does not play any role.[14] Sleep fragmentation is common in these patients even in the presence of normal sleep efficiency making the overall quality of sleep poor.[2] Among subjects suffering from malignancy, sleep disturbance has multiple effects-ranging from fatigue, depression, anxiety to immunosuppression, which in turn may worsen the quality of life and have negative impact on the course of illness.[234]
Sleep is tightly linked with the concentration of interleukin (IL)-6; relationship appears to be reciprocal and dependent on environmental time and sleep deprivation and good sleep efficiency reduce the level of IL-6 at night and following day, respectively.[567] Conversely, sleep fragmentation increased the IL-6 production by monocytes following morning.[8] IL-6 concentration is also linked with sleep stage. Non-rapid eye movement sleep Stages 1 and 2 and REM sleep have been found to increase its levels while negative correlation was seen with slow wave sleep.[56] Relationship between IL-6 and sleep appears to be bidirectional with increased IL-6 may alter nighttime sleep and also cause excessive daytime sleepiness.[7] However, sleep is not the only factor associated with IL-6 concentration in serum emotions but also modulate serum IL-6. Good social interaction lowers the daytime IL-6 level in blood, even in the presence of poor sleep efficiency and vice-versa.[7] A causal relationship was reported between elevated serum IL-6 during the day and poor sleep quality and between poor sleep quality and depressive features.[9] Even mild sleep disturbance can precipitate depressive symptoms in an environment of increased inflammation, perhaps by increasing sensitivity to cytokines, especially in women.[10]
IL-6, by acting on the IL-6 receptor, stimulates gp 130 and signal transduction, modulates cell-growth, and hence, it plays an important role in the pathogenesis of many solid as well as hematological malignancies.[11] Role of IL-6 has been reported in lymphoproliferative disorders, plasma cell tumors, multiple myeloma, and B-cell and T-cell leukemia. Its serum concentration has been found to be increased in these neoplastic conditions.[11] As already discussed, elevated level of IL-6 can worsen sleep. Sleep fragmentation has in turn, been found to worsen the tumor growth in experimental study.[12] In addition, shorter sleep duration and frequent snoring have been found to be associated with poorer survival among patients with breast cancer.[13]
Information regarding restless legs syndrome (RLS) among patients with malignancy is limited. Reported the prevalence of RLS among subjects with malignancy varies between 0.87% to 18.3%.[1415] In these patients, female gender and antineoplastic therapy for at least 3 months were predictors of RLS.[15] Patients with RLS have a higher level of anxiety and depression.[15] Conversely, higher prevalence of malignancies has also been reported among patients suffering from RLS; however, it was not related to either radiotherapy or chemotherapy.[16] For unknown reasons, RLS can also be an antecedent to the diagnosis of malignancy.[16]
However, previous literature had some limitations. Although sleep problems and mood have been examined in subjects diagnosed with cancer or cancer survivors, never before the disclosure of diagnosis to patients.[1234] Similarly, inflammatory markers, for example, tumor necrosis factor (TNF)-alpha, soluble TNF-RII, IL-1, IL-6, IL-8, and IL-10 have been examined in diagnosed patients with cancer or those undergoing any kind of the treatment.[1718] Disclosure of diagnosis and undergoing treatment for malignancy is stressful situations to most of the patients than can interfere with sleep and induce depressive features. In addition, acute as well as persistent stress and also, cytotoxic antineoplastic therapy can increase the expression of IL-6 in hypothalamus and serum.[192021] These facts suggest that IL-6 estimated before disclosing diagnosis or starting therapy would be a better representative. Third, literature assessing sleep disturbance among adult subjects with hematological malignancies is limited. Last, baseline levels of inflammatory markers have been examined as prognostic markers in solid tumors, but to the best of our knowledge its role in hematological malignancies as baseline prognostic marker is limited.[2223]
Hence, the present study was designed with the hypothesis that subjects with hematological malignancies having poor sleep quality would be having higher inflammatory load (serum IL-6) and depressive features. Moreover, we hypothesized that sleep disturbance and inflammatory load at baseline would predict 6 months survival in these patients. To overcome the limitations discussed above, baseline observations were made before disclosing the diagnosis of malignancy to study subjects.
METHODS
This study was done after obtaining approval from the Institutional Ethics committee. Subjects who visited the pathology section for bone marrow examination having either peripheral blood features of any of hematological malignancy or proven case of lymphoma by previous fine needle aspiration cytology or biopsy were included in our study. Only those cases were included in the study that was unaware of primary diagnosis. All subjects who provided written informed consent were included in this study. However, subjects having chronic inflammation, rheumatoid arthritis, tuberculosis, the recent history of fever, neurodegenerative disorder, stroke, epilepsy, psychiatric disorder or sleep disorder, having chronic obstructive lung disease, congestive heart failure, chronic pain, consuming addictive substances except for nicotine were excluded from the study. Similarly, children and pregnant women were not included in the study. Subjects who were prescribed any psychotropic medication for regular use antipsychotic, stimulants, antidepressants, dopaminergic medications were also excluded from the study. The study also included ten healthy individuals (five females and five males) and serum IL-6 levels were assessed in them.
Assessment of serum interleukin-6
Three ml of blood was drawn from antecubital vein using silica-coated plain vacutainer-needle system (BD Vacutainer, NJ, USA) after taking all aseptic precautions at the baseline and after 1 month of chemotherapy. Samples were collected between 9 am and 12 am to control for diurnal variation.
Samples were centrifuged at 1500 rpm for 10 min. Serum obtained was aliquoted in Eppendorf tubes and immediately stored at −70° in deep freezer. Serum IL-6 ELISA kit for quantitative estimation (Boster Biological Technology Limited, CA, USA) was procured maintaining the cold chain. This kit contained 96 wells, which were coated with human IL-6 specific-specific monoclonal antibodies. The human-specific detection polyclonal antibodies were biotinylated. The test sample and biotinylated antibodies were added to the wells subsequently and then followed by washing with phosphate-buffered saline or Tris-buffered saline buffer. Avidin-Biotin-Peroxidase complex was added. After washing to remove unbound conjugate, Horseradish peroxidase substrate along with a color developer was added to view the enzymatic reaction (color ranging from blue to yellow). The density of yellow color was proportional to the human IL-6 amount of sample captured in plate. This was read at 430 nm. Sensitivity of kit was <0.3 pg/ml. The kit was specific with no dateable cross reactivity was seen with other cytokines.
Assessment of sleep quality
Sleep quality was assessed using Hindi version of Pittsburg Sleep Quality Index (PSQI).[24] It is a self-response questionnaire that assesses the sleep quality over the past 1 month. It has 19 items that assess seven different aspects of sleep including sleep duration, sleep latency, daytime dysfunction, subjective sleep quality, subjective sleep efficiency, sleep disturbances, and use of hypnotic medications. Addition of scores of these domains provides a composite score. Score greater than 5 has sensitivity of 89.6% and specificity of 86.5% in differentiating between good and poor sleepers.
Assessment of depression
Depression was assessed with the help of Hindi version of Patient Health Questionnaire-9 (PHQ-9).[25] It has nine items based on the Diagnostic and statistical manual of mental disorders-4th Edition, Text Revision criteria of depression that are scored on Likert scale of 0–3. Score of 10 or more on PHQ-9 diagnose major depressive disorder with sensitivity of 88% and a specificity of 88%.
Assessment of restless legs syndrome
Restless legs syndrome was diagnosed in a face-to-face interview following standard criteria.[26] These criteria include an urge to move legs, evening worsening of symptoms, aggravation of symptoms at rest, and improvement after activity. Certain RLS mimics including habitual foot tapping, leg edema were excluded before making diagnosis of RLS.
Follow-up
All included subjects were followed for 6 months starting from the day of the enrollment in the study.
Statistical analysis
Statistical analysis was done using Statistical Package for Social Sciences SPSS v. 21.0; IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY, USA. Descriptive statistics were calculated. Chi-square was used to compare the proportions between two groups. However, wherever Chi-square was not applicable due to low cell values, Fisher's exact test was applied. Distribution of continuous variables was skewed due to small sample size, hence, Mann–Whitney U test was used to compare these variables. Binary logistic regression analysis was done to develop a model for factors contributing to poor sleep quality and survival at 6 months in this sample. Finally, the effect of individual domains of PSQI as mentioned above was also assessed in relation with overall sleep quality, depression, RLS, and survival at 6 months.
RESULTS
This study included 66 subjects with male predominance (59.1%). The average age was 50.16 + 20.42 years. Distribution of diagnostic categories is depicted in Table 1. At the time of presentation, 22.7% had clinically significant depression (PHQ score >10) and 6.1% were diagnosed as having RLS. Nearly one-third of them (28.8%) had poor quality sleep (PSQI >5) during the past 1 month. Nearly 22.7% of the subjects died at the follow-up of 6 months.
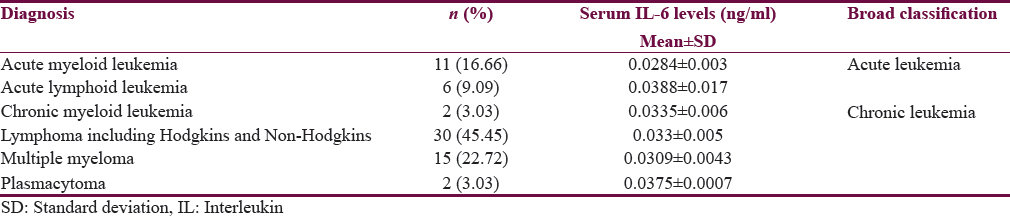
Table 2 shows the comparison of various clinical and hematological parameters with sleep quality. Different aspects of overall sleep quality including sleep duration, sleep latency, sleep disturbance, sleep efficiency, daytime dysfunction, subjective sleep quality, and use of hypnotic medications did not influence serum IL-6 concentration at baseline. However, all these domains had significant contribution to total PSQI score. The mean serum IL-6 levels for 10 healthy individuals were 0.0193 ± 0.0014 ng/ml in the study.
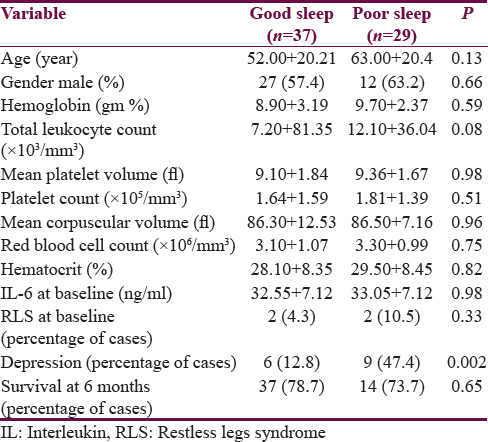
Subjects with and without RLS were comparable in relation to age, gender, type of malignancy, presence of depression, hemoglobin, mean corpuscular volume, hematocrit, and IL-6 concentration at baseline and 6 months survival.
Subjects with and without depression were comparable in relation to age, gender, diagnosis, RLS, hematological parameters level of IL-6 at baseline and survival at 6 months. However, they differ in relation to certain aspects of sleep. Sleep disturbance (P < 0.001), daytime dysfunction (P < 0.001) and use of over-the-counter hypnotic medications were more common among them.
Survival at 6 months was not influenced by gender (P = 0.26), RLS (P = 0.65), depression (P = 0.46) and overall sleep quality (P = 0.65). However, among different aspects of sleep, sleep disturbance at baseline (P = 0.01) was associated with poor survival at 6 months. Other factors did not have any influence.
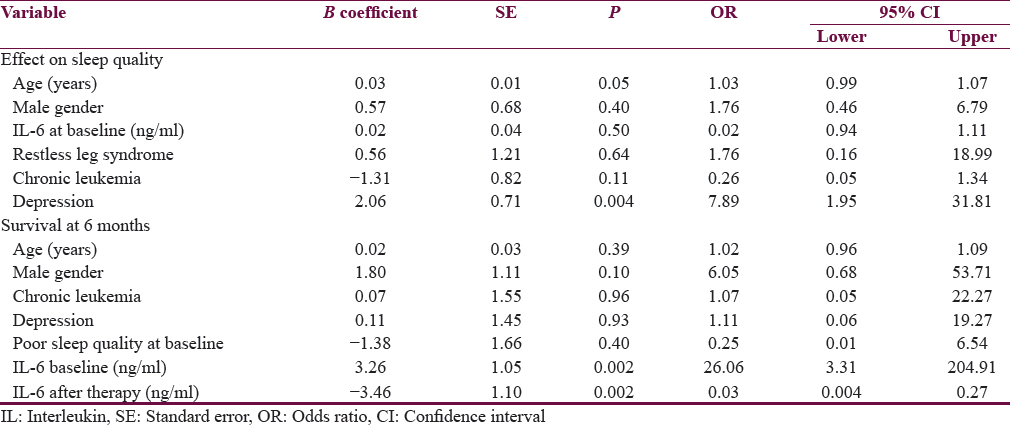
Binary logistic regression analysis was to assess the effects of various variables on sleep quality and survival. Age, gender, depression, RLS, diagnostic category (acute versus chronic), and IL-6 concentration at baseline were examined for the likelihood of having poor sleep quality. Model was overall significant (χ2 = 14.58; P = 0.02). This model explained 28% variance (Neglekerke R2 = 0.284) and correctly classified 78.8% cases. This model suggested that poor sleep quality was associated with depression [Table 3]. Model for survival at 6 months was also significant overall (χ2 = 39.06; P < 0.001). This model accounted for 68% variance (Neglekerke R2 = 0.679). It classified 90.9% cases correctly. It showed that IL-6 at baseline and after treatment predicted poor survival at 6 months.
DISCUSSION
This study reports some important results. First, subjects suffering from hematological malignancies have higher rates of depression, poor sleep quality, and RLS compared to prevalence in the general healthy population. According to the National Mental Health Survey of India 2015–2016, the prevalence of depressive disorder in Indian population is 2.7%.[27] Studies have also shown that the prevalence of sleep disorders and restless leg syndrome in general healthy population of India is 15%–19% and 2%, respectively.[282930] Our study observed higher rates of these disorders in hematological malignancies. Moreover, these features were not associated with distress of diagnosis. Second, IL-6 at baseline did not contribute to the sleep quality, depression or RLS. Third, poor sleep quality increases likelihood of having depression. In addition, three domains of PSQI-disturbed sleep, daytime dysfunction, and use of hypnotics were more prevalent among subjects with depression. Finally, sleep disturbance, higher IL-6 at baseline were associated with poor survival while IL-6 after therapy was negatively associated with survival.
Higher prevalence of sleep disturbance and depression among subjects with hematological malignancies have been reported earlier as well.[3132] Among these, sleep quality and depression are influenced by stress.[33343536] Other factors that may contribute to insomnia among cancer patients are age, gender, previous sleep history, type of cancer, severity of pain, and adverse effects of chemotherapy or bone marrow transplantation.[31] Similarly, smoking, comorbidities and environment at home have been found to predict depression in subjects with hematological malignancies, though some of the findings were contradictory across studies.[3237] IL-6 and other inflammatory cytokines have been implicated in insomnia and depression associated with malignancy and also with stress.[320] However, serum IL-6 was not found to have any contribution to these disorders in the present study sample. This could be because of two reasons, first, all included subjects were having malignancy and consequently similar serum IL-6, and in absence of healthy control group, role of IL-6 may not be ascertained. Second, it may be possible that sleep disturbance and depression might be related to IL-6 concentration in specific areas in the brain, rather than in serum.[2038] Since the potential role of distress arising out of disclosure of a diagnosis of malignancy, and its treatment has been ruled out in this study, it does not appear to be contributed to poor sleep quality and depression. However, these patients were suffering from the burden of symptoms, which could have interfered with sleep. Further, disturbed sleep and daytime distress were found to be associated with sleep in the present study. Insomnia is known to produce physical and cognitive symptoms that could mimic symptoms of depression.[39] Distress arising out of symptoms of malignancy could also have been mistaken for depression or contributed to it; however, it needs to be confirmed in a longitudinal study.
This is probably the first study to report increased prevalence of RLS among subjects with hematological malignancies which is thrice as compared to population prevalence.[40] Similar results ranging from three to nine times the prevalence in normal population have been reported earlier as well; however, these subjects were undergoing chemotherapy and reported in solid tumors.[151631] Lesser prevalence has been reported in subjects undergoing palliative care, probably related to use of opioid therapy during palliation.[14] Anemia is common in patients suffering from cancer, as also seen in the present study [Table 2].[41] Cancer leads to either absolute or functional deficiency of iron. Functional deficiency occurs due to increase in IL-6 and activation of hepcidin pathway that may lead to increased prevalence of RLS among these patients.[41]
Another important finding of the present study was role of disturbed sleep and IL-6 as prognostic markers for cancer survival. The presence of short sleep duration and snoring, before the diagnosis of cancer was made, has been found to predict poor survival among patients with breast cancer, but not other tumors.[13] Sleep deprivation activates the pro-inflammatory cytokine response of monocytes.[8] In addition, fragmented sleep accelerated the growth of tumor through activation of tumor-associated macrophages and toll-like receptor 4 (TLR4) pathway.[12] Inflammation is also associated with survival in patients having malignancy. High pretreatment IL-6 have been found with poor survival in solid tumors, a finding that was confirmed among hematological malignancies in present study.[2342] IL-6 increases the tumor growth through autocrine and paracrine manner and is also produced by malignant cells, contributing to a vicious cycle in accelerating the tumor growth.[1143] Chemotherapy is also associated with increase in inflammatory markers; however, the role of this inflammation in tumor growth is controversial.[44] On the one hand, increased IL-6 has been reported to be protective in tumor microenvironment and considered to induce resistance to therapy through various mechanisms.[4546] On the other hand, cytokines are released after chemotherapy secondary to tumor cell destruction.[45] Thus, the reduction of cytokines after chemotherapy indicates impaired drug efficacy and poor survival, as seen in the present study.
However, like other studies, the present study also had some methodological limitations. First, the sample size was small, and group was heterogeneous. In the future, studies with larger sample size are required. Second, we did not address the effect of various chemotherapeutic agents on the issue of survival. Third, questionnaire was used to diagnose depression, which could have inflated the proportion of subjects in this group. Fourth, sleep and depression should have been assessed longitudinally for better understanding of interaction between malignancy and these disorders. Another important limitation of the present study is that age-matched controls were not included and therefore the role of IL-6 in sleep disorder and depression in association with hematological malignancies cannot be ascertained. Further, larger studies with age-matched controls are therefore proposed.
CONCLUSION
The present study suggests that poor sleep quality, depression, and RLS are prevalent among adult subjects with hematological malignancies. Disturbed sleep and depression may be interlinked with additional effect from symptoms of malignancy. Sleep disturbance, high pretreatment inflammatory, and lowering of inflammatory load after chemotherapy increase likelihood for poor prognosis. Serum IL-6 did not show any association with sleep quality, restless-legs-syndrome, and depression. Further larger studies with extended follow-up are required to assess the sleep quality and depression in hematological malignancies and to study its association with inflammatory markers.
Financial support and sponsorship
This study was supported by grant from Himalayan Institute of Medical Sciences, Swami Rama Himalayan University vide letter no. HIMS/RC/2015/1060.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are also thankful to MAPI research trust for allowing us to use PSQI for this study.
REFERENCES
- Sleep problems in cancer patients: Prevalence and association with distress and pain. Psychooncology. 2012;21:1003-9.
- [Google Scholar]
- Sleep-wake disturbances in patients with advanced cancer and their family carers. J Pain Symptom Manage. 2009;38:860-70.
- [Google Scholar]
- Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester cancer center-community clinical oncology program. J Clin Oncol. 2010;28:292-8.
- [Google Scholar]
- Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci U S A. 2005;102:18757-62.
- [Google Scholar]
- Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597-603.
- [Google Scholar]
- The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun. 2005;19:165-72.
- [Google Scholar]
- Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756-62.
- [Google Scholar]
- Cytokine-induced depression during IFN-alpha treatment: The role of IL-6 and sleep quality. Brain Behav Immun. 2009;23:1109-16.
- [Google Scholar]
- Preexisting mild sleep disturbance as a vulnerability factor for inflammation-induced depressed mood: A human experimental study. Transl Psychiatry. 2016;6:e750.
- [Google Scholar]
- Impact of interleukin-6 in hematological malignancies. Transfus Med Hemother. 2013;40:336-43.
- [Google Scholar]
- Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74:1329-37.
- [Google Scholar]
- Pre-diagnostic sleep duration and sleep quality in relation to subsequent cancer survival. J Clin Sleep Med. 2016;12:495-503.
- [Google Scholar]
- Prospective evaluation of the frequency and treatment of restless legs syndrome in a palliative care unit. J Pain Symptom Manage. 2012;44:e3-5.
- [Google Scholar]
- Restless legs syndrome and its relationship with anxiety, depression, and quality of life in cancer patients undergoing chemotherapy. Qual Life Res. 2010;19:531-7.
- [Google Scholar]
- Restless legs syndrome and cancer: An analysis in three independent studies. Epidemiol Rep. 2014;2:4.
- [Google Scholar]
- Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517-22.
- [Google Scholar]
- Relationship between fatigue, sleep quality and inflammatory cytokines during external beam radiation therapy for prostate cancer: A prospective study. Radiother Oncol. 2016;118:105-11.
- [Google Scholar]
- Posttraumatic stress disorder: An immunological disorder? Front Psychiatry. 2017;8:222.
- [Google Scholar]
- Induction of IL-6 by cytotoxic chemotherapy is associated with loss of lean body and fat mass in tumor-free female mice. Biol Res Nurs. 2015;17:549-57.
- [Google Scholar]
- Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC Cancer. 2010;10:203.
- [Google Scholar]
- Interleukin-6 and C-reactive protein as prognostic biomarkers in metastatic colorectal cancer. Oncotarget. 2016;7:75013-22.
- [Google Scholar]
- The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213.
- [Google Scholar]
- The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-13.
- [Google Scholar]
- Restless legs syndrome/Willis-ekbom disease diagnostic criteria: Updated international restless legs syndrome study group (IRLSSG) consensus criteria – History, rationale, description, and significance. Sleep Med. 2014;15:860-73.
- [Google Scholar]
- National Mental Health Survey of India, 2015-16: Summary. Publication No. 128. Bengaluru: National Institute of Mental Health and Neuro Sciences (NIMHANS); 2016.
- [Google Scholar]
- Prevalence of insomnia in urban population of west Bengal: A community based cross sectional study. Int J Med Public Health. 2015;5:293-6.
- [Google Scholar]
- Sleep-related disorders among a healthy population in South India. Neurol India. 2012;60:68-74.
- [Google Scholar]
- Anxiety and depression among haematological cancer patients attending treatment centres: Prevalence and predictors. J Affect Disord. 2014;165:176-81.
- [Google Scholar]
- Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 2015;8:143-52.
- [Google Scholar]
- Clinical and biochemical manifestations of depression: Relation to the neurobiology of stress. Neural Plast 2015 2015 581976
- [Google Scholar]
- The mediating role of mental adjustment in the relationship between perceived stress and depressive symptoms in hematological cancer patients: A cross-sectional study. PLoS One. 2015;10:e0142913.
- [Google Scholar]
- Depression and anxiety in patients with hematological malignancies, prevalence, and associated factors. Saudi Med J. 2016;37:877-81.
- [Google Scholar]
- Stress activation of IL-6 neurons in the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2010;299:R343-51.
- [Google Scholar]
- The relationship between insomnia and depressive symptoms: Genuine or artifact? Neuropsychiatr Dis Treat. 2011;7:57-63.
- [Google Scholar]
- High prevalence of restless legs syndrome/Willis ekbom disease (RLS/WED) among people living at high altitude in the Indian Himalaya. Sleep Med. 2017;35:7-11.
- [Google Scholar]
- Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312-6.
- [Google Scholar]
- Inflammatory pathways as promising targets to increase chemotherapy response in bladder cancer. Mediators Inflamm 2012 2012:528690.
- [Google Scholar]
- Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther. 2014;7:1015-23.
- [Google Scholar]
- Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553-72.
- [Google Scholar]






