Translate this page into:
Ruptured Intracranial Dermoid and the Chemical Shift Artifact
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
A 25-year-old man presented with sudden onset diplopia 1 week back which had persisted. On examination, he had right lateral rectus paresis. He was investigated with a computerized tomogram (CT) which showed the right sylvian fissure hypodense mass with associated multiple small hypodense areas in the subarachnoid spaces. Further evaluation with a magnetic resonance imaging (MRI) revealed the right sylvian fissure lesion which was hyperintense on T1 and heterogeneously hyperintense on T2-weighted images with complete homogeneous suppression of the signal on fluid-attenuated inversion recovery sequence. Multiple small T1 hyperintense spots were seen in the subarachnoid spaces and inside the ventricle [Figure 1]. On susceptibility weighted imaging (SWI), the lesion appeared hypointense with blooming artifacts most prominent along its peripheral margin. The spot in the subarachnoid space also appeared hypointense with blooming and “chemical shift artifact [Figure 2].” The final diagnosis was Sylvian fissure dermoid with spontaneous rupture. The patient was conservatively managed with steroids for 3 weeks after which his diplopia completely recovered. At follow-up period of 20 months, the patient is asymptomatic.
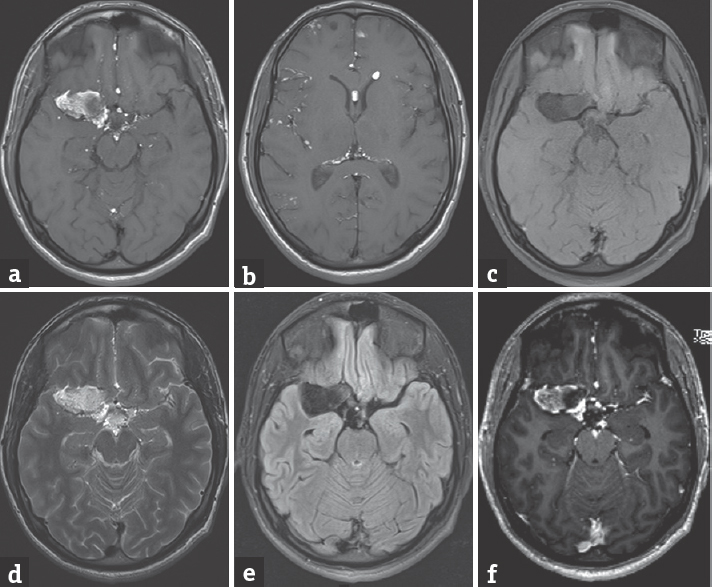
- (a) T1 image axial section magnetic resonance imaging showing the right sylvian fissure heterogeneously hyperintense lesion with multiple small lesions at anterior interhemispheric fissure, interpeduncular, and suprasellar cisterns. (b) T1 image axial section showing hyperintense fat droplets in the cavum septum pellucidum, left frontal horn, right sylvian fissure, and the subarachnoid spaces. (c) Fat saturated image showing suppression of fat in the lesion. (d) T2 image showing lesion which is heterogeneously hyperintense. (e) Fluid-attenuated inversion recovery sequence showing heterogeneous signal in the lesion. (f) Contrast-enhanced T1 image showing the absence of enhancement
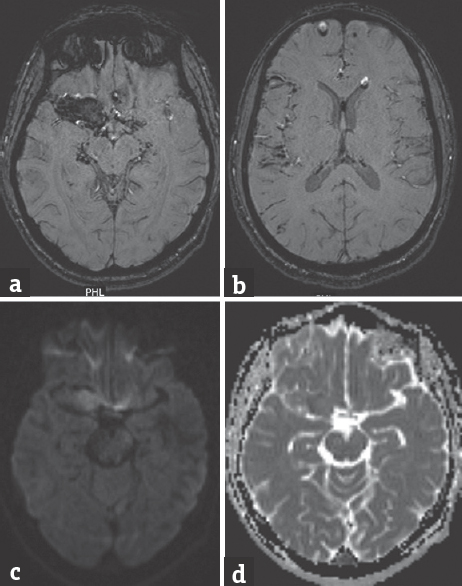
- (a) Susceptibility weighted imaging showing blooming of the lesion. (b) Fat droplets in the ventricles and frontal region show chemical shift artifact with fat-fluid levels in the nondependent portions. (c) Diffusion-weighted images show hyperintensity with. (d) Apparent diffusion coefficient showing lesion characteristic similar to brain parenchyma
Intracranial dermoids are rare congenital conditions accounting for 0.04%–0.6% of all intracranial tumors and are derived from ectopic cell remnants incorporated into the neural tube. They are composed fibrous stratified squamous epithelium capsule, enclosing thick viscous greenish-brown fluid. This fluid contains lipid metabolites, cholesterol crystals, whorls of hair, calcified areas, and decomposed epithelial cells; and occasionally sebaceous or sweat glands.[1] Occasionally, dermoid can rupture which can cause aseptic chemical meningitis due to disseminated cholesterol debris. Although spontaneous rupture is common, a head injury can precipitate rupture.[2] Rupture can cause headache, seizures, and cerebral ischemia with focal deficit.
In CT scan, ruptured dermoid appears as a smooth delineated, hypodense lesion with-50–100 Hounsfield Units with multiple small hypodense areas in the subarachnoid space. The solid lesion may show peripheral calcification. In MRI scans, dermoids appear hyperintense on T1-weighted imaging and heterogonous on T2-weighted imaging, low-fat content can appear like cerebrospinal fluid. If dermoid ruptures, fat droplets which are hypodense on CT or hyperintense on T1 in MRI will be seen scattered and floating within the nondependent portions of the ventricular system and/or subarachnoid space. Fat constituents will create a so-called “chemical shift” artifact in SWI due to misregistration of the signal in the frequency-encoded direction, which helps in radiological diagnosis.[3] Fat droplets in SWI appear hypointense and show blooming artifacts. It is more prominent along the peripheral margins of the droplets seen as a thin dark rim. As these lesions can mimic hematoma on SWI, fat-suppressed T1-weighted imaging will help to differentiate both.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Intracranial dermoid cysts: Variations of radiological and clinical features. Acta Neurochir (Wien). 2008;150:1227-34.
- [Google Scholar]
- Ruptured intracranial dermoid cyst causing headache and meningism. BMJ Case Rep 2016 2016 pii: Bcr2015213527
- [Google Scholar]
- Susceptibility artifacts in ruptured intracranial dermoid cysts: A poorly understood but important phenomenon. Neuroradiol J. 2014;27:677-84.
- [Google Scholar]





