Translate this page into:
Results of early cranial decompression as an initial approach for damage control therapy in severe traumatic brain injury in a hospital with limited resources
Address for correspondence: Prof. Andrés M. Rubiano, MEDITECH Foundation, Calle 5 #11-19, Neiva (Huila) – Colombia. E-mail: andresrubiano@aol.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Severe traumatic brain injury (sTBI) is a disease that generates significant mortality and disability in Latin America, and specifically in Colombia. The purpose of this study was to evaluate the 12-month clinical outcome in patients with sTBI managed with an early cranial decompression (ECD) as the main procedure for damage control (DC) therapy, performed in a University Hospital in Colombia over a 4-year period.
Materials and Methods:
A database of 106 patients who received the ECD procedure, and were managed according to the strategy for DC in neurotrauma, was analyzed. Variables were evaluated, and the patient outcome was determined according to the Glasgow Outcome Score (GOS) at 12 months postinjury. This was used to generate a dichotomous variable with “favorable” (GOS of 4 or 5) or “unfavorable” (GOS of 1–3) outcomes; analysis of variance was performed with the Chi-square, Wilcoxon–Mann–Whitney and Fisher tests.
Results:
An overall survival rate of 74.6% was observed for the procedure, At 12 months postsurgery, a favorable clinical outcome (GOS 4–5) was found in 70 patients (66.1%), Unfavorable outcomes in patients were associated with the following factors: Closed trauma, an Injury Severity Score >16, obliterated basal cisterns, subdural hematoma as the main injury seen on the admission computed tomography, and nonreactive pupils observed in the emergency department.
Conclusion:
Twelve months outcome of patients with sTBI managed with ECD in a neuromonitoring limited resource University Hospital in Colombia shows an important survival rate with favorable clinical outcome measure with GOS.
Keywords
Cranial decompression
damage control
traumatic brain injury
Introduction
Severe traumatic brain injury (sTBI) is a disease of global significance. The incidence of sTBI has been reported at 200 cases per 100,000 people worldwide.[1] According to the World Health Organization's study on the global burden of disease in 2010,[23] trauma remains as a public health problem and generates a significant burden on healthcare systems in Latin American countries. In Colombia in particular, the global burden of injuries is bigger in economically active, male population between 12 and 45 years of age. In 2013, for example, about 26,000 deaths resulted from trauma, and most were associated with interpersonal violence; of these injuries, a large percentage were associated with both closed and penetrating TBI.[4]
The objective of this study was to evaluate the outcomes of patients with sTBI treated with a strategy of early cranial decompression (ECD) as a damage control procedure (DC). This study was undertaken over a period of 4 years in a University Hospital in Colombia with limited neuromonitoring resources in the Intensive Care Unit (ICU). The hospital at which this study was conducted, Neiva University Hospital (NUH), is a 504-bed, level I trauma center and tertiary referral hospital in southern Colombia. NUH admits approximately 2000 adult trauma patients per year and has 30 adult ICU beds. The hospital is the primary trauma center for 3.2 million inhabitants living in an area extending over 60,000 square miles. Its radius of care extends far into the Amazonian region, where the most intense fighting between rebel groups, cocaine traffickers, and government forces has taken place for over 40 years. In this setting, TBI is exceptionally common, but few resources have been devoted to neurologic care in the hospital. NUH has one computed tomography (CT) and one magnetic resonance imaging machine and did not have continuous access to advance neuro-monitoring. Thus, this is an appropriate location to study the effects of a DC procedure that may be implemented in a timely manner without the extensive use of already limited resources.
Materials and Methods
Design
This is a descriptive observational study of head trauma patients, who were managed with ECD as a DC approach in NUH between February 2009 and February 2013. Approval from the NUH, quality improvement office and the Institutional review board of NUH was obtained prior to conducting this study.
The patient outcomes were evaluated according to the Glasgow Outcome Scale[56] (GOS) at 12 months postinjury. Based on the GOS score, a dichotomous variable divided into “favorable” (GOS 4 or 5), and “unfavorable” (GOS 1–3) groups was created. Patients were evaluated using the GOS in both the outpatient clinic and by phone interview. Classic scale scoring was used (1 = dead, 2 = vegetative state, 3 = severe disability, 4 = moderate disability, and 5 = good recovery).
Inclusion and exclusion criteria
We included in the study only those patients managed with an ECD. Additional criteria include age ≥18 years old, severe head trauma (Glasgow Coma Score ≤8 on arrival or head Abbreviated Injury Scale ≥3) and ICD-10 diagnostic codes of S-00 to S-09 or T-00 to T-14. All of the patients included in the study were operated in less than 12 h post-trauma. We excluded patients with severe extracranial traumatic injuries, patients who do not receive a decompressive craniectomy, and patients operated after 12 h posttrauma.
Early cranial decompression technique
Early decompression included a >12 cm by 12 cm hemispheric craniectomy[7] either with or without dural closure. Surgical criteria for the procedure included: Obliteration of the basal cisterns, midline shift of >0.5 cm, acute subdural hematoma wider than 1cm, epidural hematomas of >30 cc in volume, or intracerebral hemorrhage of >50 cc in volume [Figure 1].
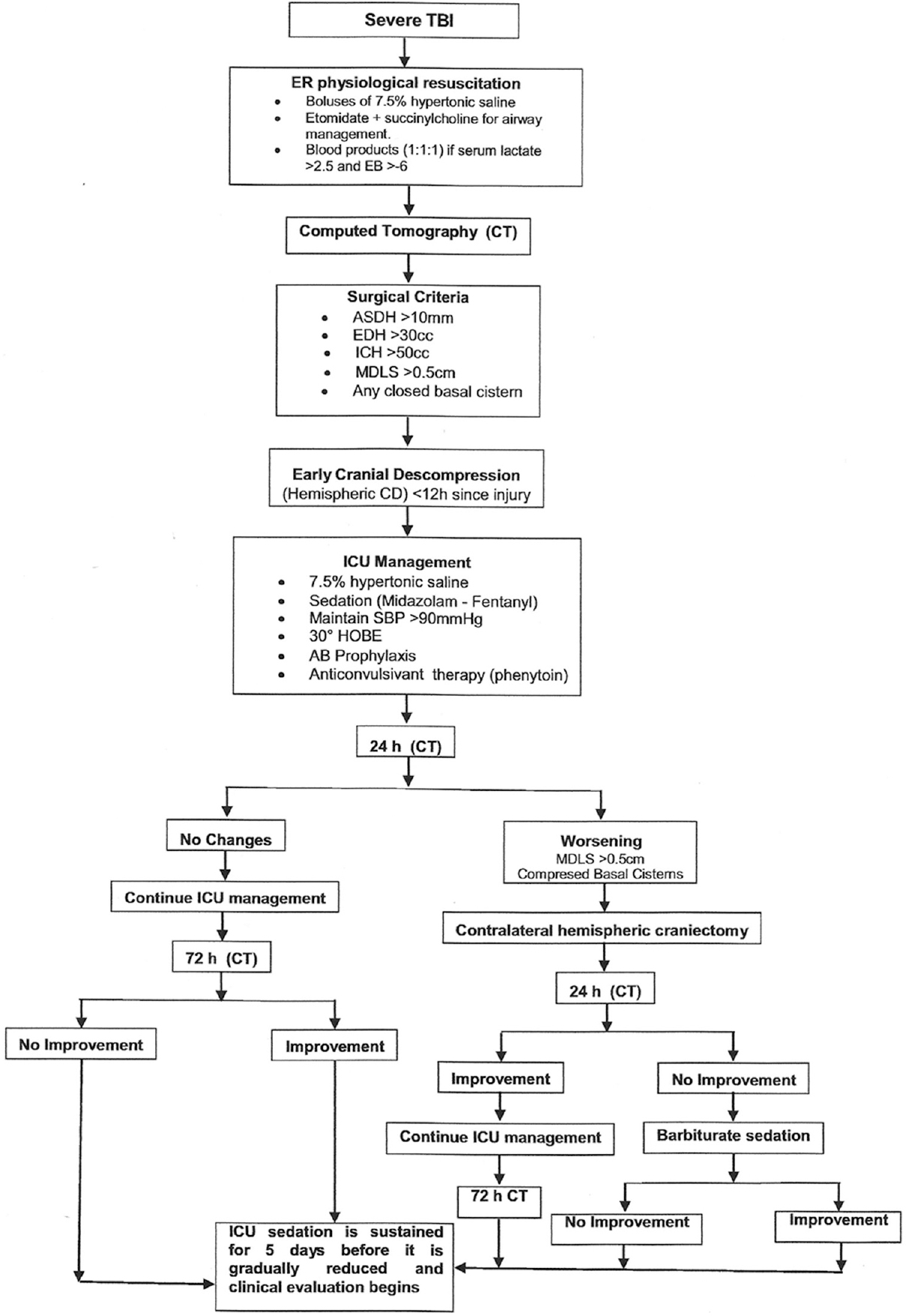
- Algorithm for management of severe TBI patients at Neiva University Hospital. TBI: Traumatic brain injury, ER: Emergency room, EB: Excess of base, ASDH: Acute subdural hematoma, EDH: Epidural hematoma, ICH: Intracranial hemorrhage, MDLS: Midline shift, CD: Cranial decompression, ICU: Intensive care unit, SBP: Sistolic blood pressure, HOBE: Head of the bed elevation, AB: Antibiotic
Postoperative care
Postoperative care includes sedation for at least 5 days with midazolam and fentanyl, 7.5% hypertonic saline in boluses every 6 h for 48 h and control CT at 24 h after surgery. Head of the bed elevation at 30° was used. Antibiotic and anti-convulsive prophylaxis was used in penetrating injuries [Figure 1].
Data collection and statistical analysis
Documentary review of medical records by data recording was performed using an intake form that included epidemiological, clinical, surgical, and outcomes data. The analysis of clinical, demographic, and imaging variables was performed for patients who had TBI and were operated with an ECD. Variables such as Glasgow Coma Scale at the emergency room, type of trauma, the severity of injuries, CT scan findings including the presence of hematoma, midline shift, and the compression of the basal cisterns were included. The outcome was evaluated with GOS. The results obtained in the study were analyzed by a statistical R Software, Version 2.15.2, R Foundation, Free Software Foundation (Boston – MA), USA. Measures of central tendency and dispersion for continuous variables were calculated including frequencies and proportions for categorical variables. The Student's t-test was used to compare continuous variables, and Pearson Chi-square test was used for categorical variables
Results
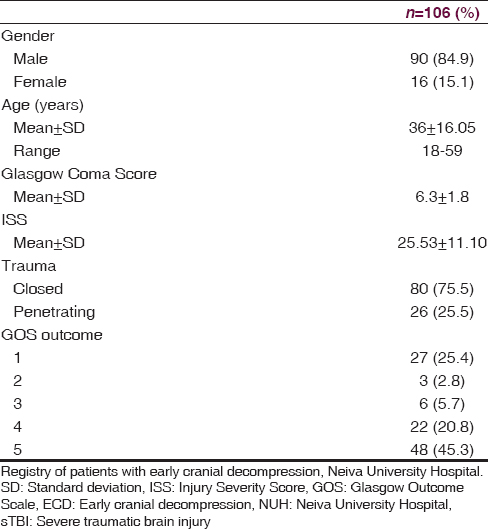
At NUH, 156 patients were admitted with a diagnosis of sTBI between February 2009 and February 2013, but only 106 were managed under ECD with all the inclusion criteria [Table 1]. At 12 months postsurgery, a favorable clinical outcome (GOS 4–5) was found in 70 patients (66.1%), while an unfavorable clinical outcome (GOS 1–3) was found in 36 patients (33.9%) (P = 0.0001). Of the 36 patients with an unfavorable outcome, mortality (GOS = 1) was observed in 27, with an overall rate of 25.4%. 70.1% (20) of the patients who die, were patients admitted for penetrating brain injury. The clinical and demographic characteristics of both groups are described in [Table 2].
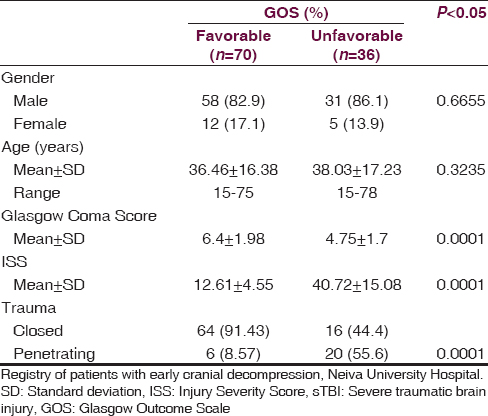
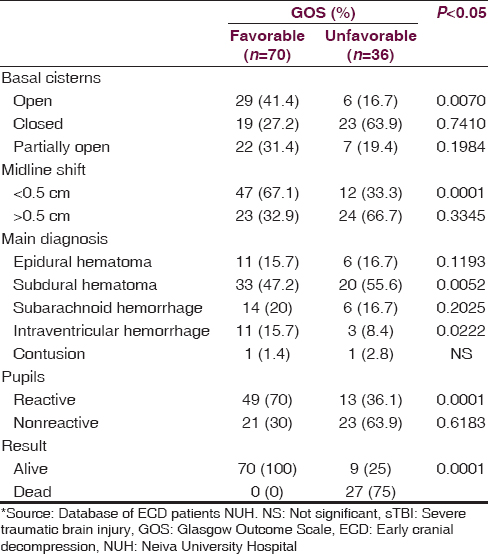
The factors that were associated with an unfavorable neurologic outcome were the following Injury Severity Score (ISS) >35.62 (95% confidence interval [CI], 35.6–45.8), subdural hematoma at the first CT, closed basal cisterns, and unreactive pupils upon emergency room arrival. In [Table 3], significant findings were analyzed for both groups. The average length of stay in the ICU for the patients with a favorable GOS (4–5) was 12.96 ± 2.67 days while the group with an unfavorable GOS (1–3) spent an average of 26.71 ± 5.35 days in the ICU (P = 0.0002). The average total hospital stay for the favorable group was 26.60 ± 5.78 days while the unfavorable group spent 48.07 ± 12.92 days (P = 0.0001).
Postoperative care of all the patients was performed in the ICU and included sedation with midazolam and fentanyl for a mean of 5 days and 7.5% hypertonic saline boluses every 6 h for 48 h. CT imaging was performed at 24–72 h. Brain swelling was present in 100% of the cases at both time points. A control CT around day 5 was performed in all patients. Cranioplasty was performed in most of the cases with autologous bone in a mean period between 1 and 3 months after the initial decompression.
Discussion
TBI is a severe medical and surgical disease of major importance globally.[8] The World Health Organization predicts that traffic accidents will be the third leading cause of illness and injuries worldwide by 2020, and this is one of the most common causes of TBI.[9] In our study, we observed a population of 106 patients with sTBI managed with ECD, where 84.9% were male, and the mean age was 36 years, representing the population that is at a major risk for trauma in low and middle-income countries.
The management of TBI with ECD has been the subject of many studies in recent years, but often these studies do not provide enough scientific evidence for the procedure.[101112] These studies, though, utilize small sample sizes of patients, or use a time definition for ECD as >12 h posttrauma, which disregards the importance of early intervention in regards to the ECD procedure and treatment for sTBI. In addition, the results of these studies show high variability in patient age, type of surgery, and time to initiation of EDC surgery.[131415] Despite this, in many parts of the world ECD surgery as DC therapy has begun to play a critical role as management for neurotrauma.[1617] ECD has been cataloged as an important option to improve survival and reduce disability associated with TBI.[181920] This trend has been confirmed in our study where we found that of the 106 patients with sTBI receiving ECD, 79 (74.6%) survived and of those 79 surviving patients, 88.6% had a favorable neurological outcome (GOS 4–5) at 12 months after injury. We do not compare results with patients operated after 12 h in our center or with patients without surgical management in order to avoid bias into the final analysis. In addition, this sample has a homogeneous postoperative care as they were managed inside the specifically described algorithm. Contralateral decompressive craniectomy was not significant as this was used only in few patients. We do not include the other variables of the postoperative care, as part of the analytical model for the outcome, and maybe this is a limitation for this study.
Several studies of ECD have been conducted in centers where there is the availability of continuous medical monitoring in the Neurological ICU.[1516171819] In our hospital, continuous neuromonitoring in the ICU is not an option; however, the results obtained using EDC as DC therapy reflected a better neurological outcome in patients with severe head injury. Recent studies using the same technique have shown mixed results, including studies in centers of similar resources.[202122] In our study, patients who underwent ECD as DC therapy, an unfavorable neurological outcome was associated with closed trauma, an ISS > 16, obliterated basal cisterns, subdural hematoma as the predominant finding on CT, and nonreactive pupils upon admission. Many of these factors have also been described in other studies that recommend ECD as a DC therapy for sTBI,[2324] but our study findings need to be highlighted in a context of few resources for neuromonitoring and an aggressive surgical care as an option due to this resource limitation. The only available study of ECD in low resources environment includes the analysis of 10 patients in a teaching hospital in Africa.[25] At present, the management of patients with sTBI should be aggressive from the moment they arrive at the emergency department; different management protocols can be used help increase survival and reduce hospital time for these patients.[252627] In our study, hospital stay was significantly shorter for the group of patients with a favorable neurological outcome at 12 months from injury.
Conclusions
Twelve months outcome of patients with sTBI managed with ECD in a neuromonitoring limited resource University Hospital in Colombia shows an important survival rate with favorable clinical outcome measure with GOS.
Financial support and sponsorship
Authors are supported by the NIH-FIC Grant # R21TW009332 awarded to MEDITECH Foundation (COL) and the University of Pittsburgh (USA).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Authors acknowledge the Neurosurgery Service at Neiva University Hospital, MEDITECH Foundation Research Group and Social Development, Public Health and Human rights Research Group of South Colombian University for the Support in the Patient Management and Research Process.
References
- Reflections on the global burden of disease 2010 estimates. PLoS Med. 2013;10:e1001477.
- [Google Scholar]
- Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J Neurotrauma. 1998;15:573-85.
- [Google Scholar]
- The bigger, the better?. About the size of decompressive hemicraniectomies. Clin Neurol Neurosurg. 2015;135:15-21.
- [Google Scholar]
- Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24:1-106.
- [Google Scholar]
- Effect of early bilateral decompressive craniectomy on outcome for severe traumatic brain injury. Turk Neurosurg. 2010;20:382-9.
- [Google Scholar]
- Value of early unilateral decompressive craniectomy in patients with severe traumatic brain injury. Ulus Travma Acil Cerrahi Derg. 2010;16:119-24.
- [Google Scholar]
- Efficancy of decompressive craniectomy in treatment of severe brain injury at the Rijeka University Hospital Centre. Coll Antropol. 2011;35(Suppl 2):255-8.
- [Google Scholar]
- Decompressive craniectomy for severe traumatic brain injury: Evaluation of the effects at one year. Crit Care Med. 2003;31:2535-8.
- [Google Scholar]
- The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287-92.
- [Google Scholar]
- Effect of the modified Glasgow Coma Scale score criteria for mild traumatic brain injury on mortality prediction: Comparing classic and modified Glasgow Coma Scale score model scores of 13. J Trauma. 2011;71:1185-92.
- [Google Scholar]
- Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(58 Suppl):S16-24.
- [Google Scholar]
- Early decompressive craniectomy for neurotrauma: An institutional experience. Ulus Travma Acil Cerrahi Derg. 2009;15:28-38.
- [Google Scholar]
- Early decompressive craniectomy for severe penetrating and closed head injury during wartime. Neurosurg Focus. 2010;28:E1.
- [Google Scholar]
- Study of the long-term results of decompressive craniectomy after severe traumatic brain injury based on a series of 60 consecutive cases. ScientificWorldJournal 2014 2014:207585.
- [Google Scholar]
- Outcomes of patients with severe traumatic brain injury who have Glasgow Coma Scale scores of 3 or 4 and are over 65 years old. J Neurotrauma. 2010;27:1549-55.
- [Google Scholar]
- Significance of intracranial pressure monitoring after early decompressive craniectomy in patients with severe traumatic brain injury. J Korean Neurosurg Soc. 2014;55:26-31.
- [Google Scholar]
- Early decompressive craniectomy for traumatic brain injury in resource poor centres: A tertiary institution experience. Niger Postgrad Med J. 2015;22:45-9.
- [Google Scholar]
- Outcomes of decompressive craniectomy in patients after traumatic brain injury. Crit Care Resusc. 2015;17:67-72.
- [Google Scholar]
- Guidelines for the Management of Head Injuries and Head Gunshots Wounds in Combat Areas. In: Rubiano AM, Pérez R, eds. Neurotrauma and Neurointensive Care (1st ed). Bogotá: Distribuna Editorial; 2007. p. :231-44.
- [Google Scholar]
- The management of patients with intradural post-traumatic mass lesions: A multicenter survey of current approaches to surgical management in 729 patients coordinated by the European brain injury consortium. Neurosurgery. 2005;57:1183-92.
- [Google Scholar]
- Improving trauma care in low- and middle-income countries by implementing a standardized trauma protocol. World J Surg. 2014;38:1869-74.
- [Google Scholar]
- A standardized trauma care protocol decreased in-hospital mortality of patients with severe traumatic brain injury at a teaching hospital in a middle-income country. Injury. 2014;45:1350-4.
- [Google Scholar]






