Translate this page into:
Relationship between resting and action tremors in Parkinson's disease
Address for correspondence: Dr. Mohamad Saleh, 13278 Tecumseh Road E, Unit 100, Tecumseh, Ontario N8N 3T6, Canada. E-mail: dr.saleh@att.net
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To determine the relationship between resting tremor (RT) and action tremor (AT) in Parkinson's disease (PD) patients.
Methods:
A retrospective study of RT and AT severity was conducted in 100 PD patients. The severity rating for each type of tremor in the upper extremities was assessed. The disparity in tremor severity between extremities for each tremor type was compared to that of the other two to identify commonalities in the laterality of the tremor manifestation.
Results:
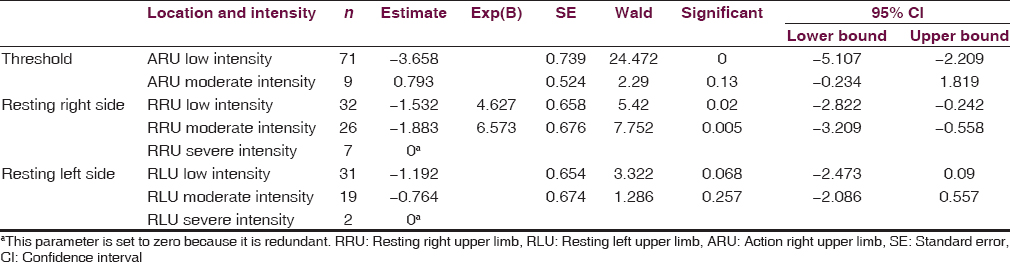
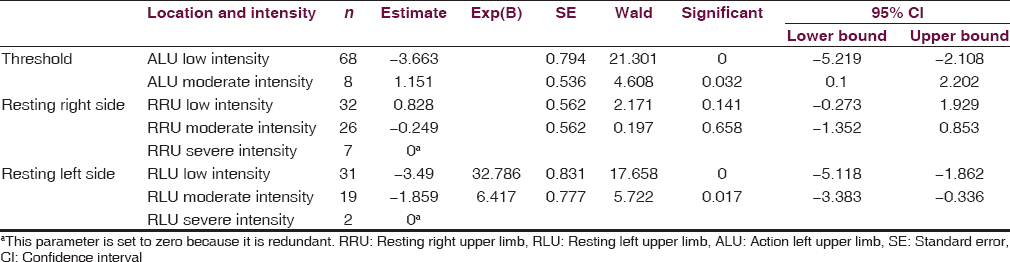
Overall, RT is predictive of AT on the same side, but not the opposing side of the body. Patients with less intense resting right upper limb (RRU) tremor and moderately intense RRU tremor were significantly more likely to have an action right upper limb (ARU) tremor (−1.53, P = 0.020; −1.88, P = 0.005, respectively). Similarly, patients with less intense resting left upper limb (RLU) tremor and moderately intense RLU tremors were significantly more likely to have an action left upper limb (ALU) tremor (−3.49, P = 0.000; −1.86, P = 0.017, respectively). In addition, RRU and ALU tremors were associated with an increase in RLU and ARU tremors, respectively.
Conclusion:
Tremors are common findings in PD patients, and often impair quality of life. By identifying and classifying the relationship between resting and ATs in PD patients, our study sheds light onto the importance of better understanding and future management of this debilitating symptomology.
Keywords
Action tremor
movement disorders
Parkinson's disease
resting tremor
Introduction
Tremors, the most common movement disorder, are defined as the rhythmic back and forth motion of a body part.[12] Much progress has been made in recent years to better elucidate tremor etiology, pathophysiology, diagnosis, and management.[1] Overall, there exist five main classifications of tremor, the choice of which depends on the associated behavior of the patient.[3] This includes resting, postural, kinetic, intention, and task-specific.[4] Resting tremors (RTs) occur when the limb is fully supported and relaxed, postural is when the limb is positioned against gravity, kinetic can be during any type of movement, intention is worse as one nears the end of a goal-directed movement, and task-specific is only during highly skilled activities.[4]
While RT is classically coupled to a diagnosis of Parkinson's disease (PD), postural and kinetic tremors are frequently seen in patients with essential tremor (ET).[3] However, it is not uncommon for patients with other conditions to present with tremor. Etiologies of pathologic tremor include, but are not limited to Wilson's disease; peripheral neuropathies (e.g., multiple sclerosis, diabetes); diseases of the cerebella and midbrain; as well as drug side effects (e.g., opioids).
PD is a progressive neurodegenerative movement disorder consisting of the following four cardinal features: Tremor, rigidity, akinesthesia/bradykinesthesia, and postural instability.[5] Patients may also complain of nonmotor symptoms, such as dementia, depression, and pain: Especially in advanced stages of disease progression. Tremor is the most common initial finding in patients with PD and is present in up to half of patients at the time of diagnosis.[5] The most traditional manifestation of tremor is an asymmetric RT, described as “pill rolling” in appearance.[6] However, recent studies have looked into the presence of postural and kinetic tremors in PD patients.[47] Of additional interest is any association between action tremor (AT), which is the combination of kinetic and postural tremors, and RT in PD patients. The current consensus on the subject is controversial, with studies by Louis et al. demonstrating a relationship, and Adler et al. arguing against one.[89] The RT in PD is difficult to manage, and may respond to treatments with carbidopa and levodopa, anticholinergic, or in extreme cases, stereotactic thalamotomy.[4] If present, the AT may sometimes improve upon the administration of propranolol.[4]
ET is defined as a syndrome with a slowly progressive postural and/or kinetic tremor.[4] Both arms are normally affected, and a distinctive feature is the presence of a head tremor. ET is the most commonly diagnosed movement disorder.[4] The etiology of ET is unknown, although there does seem to be a genetic component.[49] As mentioned earlier, some studies have supported the role of ET being a risk factor for the onset of PD, although this remains controversial.[8] Since ET rarely causes disability, medical treatment is in general not necessary. However, beta-adrenergic-receptor antagonists, such as propranolol hydrochloride, are the first-line agents when indicated.[4] Second-line agents include metoprolol in the presence of asthma; nadolol for avoiding central nervous system (CNS) side effects; primidone, which is anticonvulsant; and stereotactic thalamotomy or thalamic stimulation in refractory cases.[4]
Although the CNS origin of pathological tremors is well established, the exact mechanism and location of these central oscillators remain unclear.[1] Studies have most consistently shown the presence of multiple central oscillators throughout the basal ganglia (BG) and cerebello-thalamo networks.[10] Indeed, a combination of different cortical and subcortical motor centers has been shown to generate rest, postural, and kinetic tremors.[11]
Overall, the pathophysiology of RT in PD patients remains unclear, as aspects of it seem to contradict the dopamine-deficient etiology of bradykinesthesia and rigidity.[1213] Similarly, dysfunction of the BG is more closely associated to akinesthesia and rigidity than it is with tremor. Emerging evidence suggests the involvement of the cerebello-thalamo-cortical circuit in the underlying pathology of RT.[13] It is generally accepted that RT in PD mainly results from a central mechanism, as there are no suppression effects from peripheral differentiation.[13] Current evidence strongly suggests a number of independent oscillating circuits within cortical, subcortical, spinal, and even peripheral limb centers as the root for PD tremor.[112]
There is much interest in elucidating a relationship between RT and AT in PD patients, as is reflected by the plethora of studies with inconsistent findings. Some studies support the view that AT and RT are related while others suggest they are distinct from one another. In our study, PD patients were assessed retrospectively for the severity and correlates of resting and AT. By evaluating the relationship between resting and AT we hope to draw a clearer understanding on this confusing, yet relevant topic.
Methods
Subjects
A retrospective chart analysis of 157 patients was performed. Participants were diagnosed with idiopathic PD and were aged between 43 and 99 years, with a mean age of 75 years. Of these, 100 had a tremor and were included within our study. More specifically, 65 had rested right upper limb (RRU) tremor, 52 had resting left upper limb (RLU) tremor, 80 had an action right upper limb (ARU) tremor, and 76 had an action left upper limb (ALU) tremor. The breakdown regarding Unified PD Rating Scale (UPDRS) intensity classification can be found on Tables 1–4. Patients were diagnosed and regularly followed by a clinical neurologist with movement disorder training in a community-based PD and movement disorders center between 2005 and 2010. All patients were informed about the nature of the study and gave their written consent for participation. The local ethics review board approved the study.
Parkinson's disease diagnosis
Patients were diagnosed with PD via the UK PD Society Brain Bank clinical diagnostic criteria.[14] Patients presenting with an atypical variant of PD were excluded from this study. Patients were administered anti-PD medication at the time of data collection. This includes dopaminergic medications, monoamine oxidase-B inhibitors, amantadine, or a combination-dosing regimen. None of the participants were using beta-blockers or primidone.
Tremor diagnosis
RT occurs when the affected limb is supported by the body or an object and is not opposing gravity.[4] Patients were examined for RT in complete repose while sitting on the examination table with legs and arms relaxed, or when in the supine position. In addition, the patient's upper limbs were assessed for RT while walking during gait assessment. RTs were elicited via coactivation techniques. ATs manifest during voluntary muscle activation, and can be further classified as kinetic, intention, or task-specific.[4] Participants were asked to sit with their arms outstretched while drawing a spiral with their hands to assess for kinetic tremor. Finger-to-nose coordination was used to evaluate for intention tremor. If the tremor worsened as the patient's finger approached that of the examiners, this was positive for intention tremor. Finally, postural tremors present when the affected limb is opposing gravity and it not supported by the body or an object.[4] Postural tremor was assessed with the patient seated; both arms outstretched, and fingers spread apart.
Study parameters
Patients were initially assessed for the presence or absence of all tremor types. Tremors were further assessed for type and location. Analysis of tremor location was limited to upper extremities for ease of observation, and because this is the most prevalent location in PD populations.[2] The primary focus of this manuscript was to identify and classify the relationship between action and RTs. The severity rating for each type of tremor in each extremity was based on the respective UPDRS scores.[15] These were classified as being absent or less intense (1), moderately intense (2), or severely intense (3 and 4). UPDRS scores of 3 and 4 were combined as a result of a low sample size for the latter. For analysis, threshold values of low and moderately intense tremors were combined and measured against action and RTs of various location and intensity. The analysis was conducted to identify the predictive power of RT severity of one or both limbs on the presence and severity of AT of one or both limbs. The disparity in tremor severity between extremities for each tremor type was compared to that of the other two to identify commonalities in the laterality of the tremor manifestation.
Statistical methods
Patients were assessed for the presence of resting or AT in both upper extremities. Odds ratios and likelihoods (estimates), as well as correlations (Wald), were sought among tremor types and locations using SPSS version 19 (SPSS Inc., Chicago, IL, USA). In addition, the level of asymmetry between RT of the right and left upper extremity, as well as AT of the two extremities was examined with correlational statistics. Significance levels of 0.05 were used throughout all of the analyses.
Results
Overall
There were four main arms of this study, which altogether suggest differing etiologies between RT and AT. These analyses include relationships between ARU tremor and RRU as well as RLU tremors; correlations between ALU tremor and RRU as well as RLU; measuring the relationship between RLU and RRU, and determining the association between ARU and ALU.
Action tremor versus resting tremor
After comparing RT with AT, it was found that RT is predictive of AT on the same side of the body, whereas RT and AT on opposite sides have no predictive value on each other [Tables 1 and 2]. Indeed, patients with less intense RRU and moderately intense RRU were significantly more likely to have an ARU tremor [−1.53, P = 0.020; −1.88, P = 0.005, respectively; Table 1]. Similarly, patients with less intense RLU and moderately intense RLU tremors were significantly more likely to have an ALU tremor [−3.49, P = 0.000; −1.86, P = 0.017, respectively; Table 2]. However, patients with lesser and moderately intense RLU and RRU tremors were not more likely to have an ARU or ALU tremor, respectively. Further analysis was conducted and found that as RLU tremor intensity increased, so did the AT of the same side. The same is true vice versa. Indeed, patients with less intense RLU tremor were 32.79 times less likely to experience a more intense ALU tremor, when compared to individuals experiencing very intense RLU tremor. In addition, patients with moderately intense RLU tremor were 6.42 times more likely to report less intense ALU tremor than those with higher intensity RLU tremor. Similar trends were elucidated for right-sided tremors, except with less magnitude. Subjects with little RRU and moderate RRU tremors were 4.63 and 6.57 times more likely to have a lower intensity ARU tremor than those with a higher intensity RRU tremor, respectively.
Right side versus left side
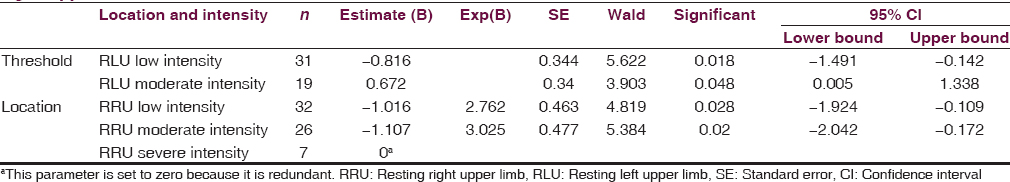
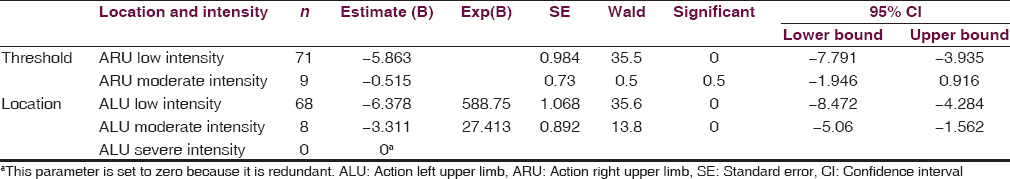
RLU and ARU tremors were assessed for any relationship to RRU and ALU tremors, respectively [Tables 3 and 4]. RLU tremors increased the prevalence of lesser intense RRU [−1.02, P = 0.028; Table 3] and moderately intense RRU tremors [−1.11, P = 0.020; Table 3]. Likewise, the presence of low or moderately intense ARU tremors was predictive of low-intensity ALU tremors [−6.38, P < 0.05; Table 4] and moderately intense ALU tremors [−3.31, P < 0.05; Table 4]. Overall, the association was stronger for ATs than RTs. It was also found that patients with little or no RT on the right are 2.76 times less likely to report higher ratings of RT on the left when compared to those with higher RT on the right. Those with moderate RTs on the right are 3.03 times less likely to report higher ratings of RTs on the left when compared to those experiencing higher RTs on the right. The probabilities for predicting RLU from RRU remain somewhat steady, and the estimates fall within the 95% confidence interval, indicating that a change in RT on one side does not cause changes in RT on the opposite side. Those with initial or moderate stages of AT on the left side are 588.75 and 27.41 times less likely to report a higher rating of AT on the right as compared to those with very intense AT on the left, respectively. Overall, there is a larger discrepancy between the probabilities of different stages of AT on one side in predicting AT on the opposite side than with RT. This suggests a link between the two sides for AT but not for RT.
Discussion
Most studies have focused on PD RT. However, as our results indicate many PD patients also have AT, which is probably an inherent symptom of the disease. Our data reveal that RT is predictive of AT on the same side, but not the opposing side of the body. The results demonstrate that when RT was present and became more severe in intensity, the occurrence of AT decreased. In addition, the presence of RRU increased the likelihood of RLU. In contrast, the presence of ALU increased the likelihood of ARU.
Our study highlights the importance of the co-occurrence of resting and AT. Although the RT is well studied and documented in PD, it is the AT, however, which is often more disabling for the patient. Patients with kinetic tremor can be handicapped in everyday situations, such as drinking from a glass, grasping an object, writing and other fine motor skills. In contrast to RT, which may represent a social handicap, AT associated with PD is directly correlated with motor disability[16] and contributes to weakness and bradykinesia.[1718]
An early study on AT in PD by Lance et al.[19] noted the presence of AT during voluntary movements and showed considerable evidence for the dissociation of AT and RT. Their results showed that PD patients with AT did not have RT and vice versa. This study also noted that ATs have a higher frequency than RT and the two do not share any harmonic relationship. Although this evidence suggests a dissociation of AT and RT, Sung et al.[20] findings showed that dopaminergic treatment did indeed affect RT but only for PD patients with moderate bradykinesia and rigidity rather than patients with other prominent parkinsonian features. Our study showed that PD patients with RT were highly likely to have AT on the same side of the body, suggesting that individuals with AT will have both the postural and kinetic components along with RT.
Teräväinen and Calne[21] suggested that the pathophysiology of AT may be similar to that of RT. Since during voluntary movement different pathways through the BG are activated compared to the resting state,[22] the occurrence of rest and AT may mainly be determined by the pathways involved in different brain states and the involvement of these pathways in the degenerative processes. From the relatedness between rest and AT, it is likely that pathways may overlap, and that both tremor types share a common pathophysiology. The relationship between neuronal activity patterns in the parkinsonian BG-thalamocortical and the cerebellothalamocortical loop, and the occurrence and severity of rest and AT, as well as the relationship with the other motor symptoms, merits further investigation through simultaneous recordings in different nuclei and movement registration, possibly combined with functional imaging techniques.
Patients were not withdrawn from medication, and the time of medication intake with respect to the measurement session may have been different for the different patients. We expect that medication may have influenced the severity and duration of tremor (both rest and AT), adding to the heterogeneity of the group. Strengths of our study include the large sample size, typical age range, and the presence of a variety of parkinsonian features in our patients. The early studies of Lance et al.[19] and Raethjen et al.[23] included a small number of PD patients who were of younger age. The association of AT and RT in our study support the findings of another study by Louis et al.[8] that looked at the clinical correlates of AT in PD. There is also evidence to suggest that resting and AT share neural substrates.[24] Although our study highly supports that the two tremors are closely related, further research that includes a control group and brain imaging is still required to completely understand the exact neurological basis of this relationship.
Conclusions
AT was associated with RT in PD, suggesting that, at least in part, AT is a manifestation of the underlying BG disease. Therefore, more attention should be paid to kinetic tremor in PD, as it is frequently present and quite disabling with respect to important everyday tasks, such as writing. A better understanding of pathophysiologic and pharmacologic processes can be expected from a more precise differentiation of the different tremor forms at PD, thereby helping those with this debilitating disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Bassel Rashid for his help in producing this paper.
References
- Assessment of tremor activity in the Parkinson's disease using a set of wearable sensors. IEEE Trans Inf Technol Biomed. 2012;16:478-87.
- [Google Scholar]
- Assessment of rest tremor in Parkinson's disease. Neurol Neurochir Pol. 2008;42:12-21.
- [Google Scholar]
- Kinetic tremor in Parkinson's disease – An underrated symptom. J Neural Transm (Vienna). 2006;113:845-53.
- [Google Scholar]
- Clinical correlates of action tremor in Parkinson disease. Arch Neurol. 2001;58:1630-4.
- [Google Scholar]
- Essential tremor and Parkinson's disease: Lack of a link. Mov Disord. 2011;26:372-7.
- [Google Scholar]
- Temporal evolution of oscillations and synchrony in GPi/muscle pairs in Parkinson's disease. J Neurophysiol. 2005;93:1569-84.
- [Google Scholar]
- Cortical involvement in the generation of essential tremor. J Neurophysiol. 2007;97:3219-28.
- [Google Scholar]
- Differentiating phase shift and delay in narrow band coherent signals. Clin Neurophysiol. 2008;119:1062-70.
- [Google Scholar]
- Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181-4.
- [Google Scholar]
- Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The unified Parkinson's disease rating scale (UPDRS): Status and recommendations. Mov Disord. 2003;18:738-50.
- [Google Scholar]
- Tremors in Parkinson's disease: Symptom analysis and rating. Clin Neuropharmacol. 1994;17:303-14.
- [Google Scholar]
- Does parkinsonian action tremor contribute to muscle weakness in Parkinson's disease? Brain. 1997;120(Pt 3):401-8.
- [Google Scholar]
- The relation between EMG activity and kinematic parameters strongly supports a role of the action tremor in parkinsonian bradykinesia. Mov Disord. 2001;16:47-57.
- [Google Scholar]
- Action tremor and the cogwheel phenomenon in Parkinson's disease. Brain. 1963;86:95-110.
- [Google Scholar]
- Factors predicting response to dopaminergic treatment for resting tremor of Parkinson's disease. Mov Disord. 2008;23:137-40.
- [Google Scholar]
- Action tremor in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1980;43:257-63.
- [Google Scholar]
- Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. Neuroimage. 2011;55:1728-38.
- [Google Scholar]
- Parkinsonian action tremor: Interference with object manipulation and lacking levodopa response. Exp Neurol. 2005;194:151-60.
- [Google Scholar]
- Parkinson's disease tremor-related metabolic network: Characterization, progression, and treatment effects. Neuroimage. 2011;54:1244-53.
- [Google Scholar]






