Translate this page into:
Rare Case of Sellar and Suprasellar Metastasis from Ewing's Sarcoma of Tibia
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We hereby report a case of metastatic Ewing's sarcoma presenting with rapid-onset total ophthalmoplegia, optic atrophy, and right temporal hemianopia. Comprehensive ophthalmic and neurological evaluation with targeted radioimaging revealed a tumor mass centered over the sella, compressing optic chiasma, extending to involve the left cavernous sinus and the left orbital apex. Whole-body imaging revealed the evidence of multifocal lung and mediastinal metastasis with focal lytic defect in the left femoral head. Histopathological evaluation of transnasal punch biopsy from the nasopharyngeal extension of the tumor revealed small round-cell tumor with strong CD99 positivity, supporting the diagnosis of Ewing's sarcoma. Rapid, aggressive extensions of the metastatic tumor into vital structures despite the initiation of chemoradiation of the extensive intracranial tumor led to unexpected demise of the patient. Our case is an unusual case of Ewing's sarcoma metastasis manifesting as a sellar mass and mimicking a pituitary adenoma radiologically, with a rapid progression within 2 weeks to cause massive extension of tumor into suprasellar, infrasellar, and left parasellar area, indicative of highly malignant nature of the tumor.
Keywords
Multimodal approach
sellar and suprasellar metastasis of Ewing's sarcoma
strong CD99 positivity
subclinical metastasis
total ophthalmoplegia
INTRODUCTION
Ewing's sarcoma, primarily a bone lesion, occurs between the first and second decades of life in 80% of cases.[12] It generally occurs in the lower extremities and pelvic girdle and rarely in the orbit. Central nervous system (CNS) involvement in case of bone or soft-tissue sarcomas is rare.[34567] There is heterogeneity in reported rates of CNS metastases in Ewing's sarcoma. Weins and Hattab[3] reported CNS metastases in 2% of cases of Ewing's sarcomas/primitive neuroectodermal tumors (PNET). Bekiesinska-Figatowska et al.[4] reported brain metastasis in 1% of cases of Ewing's sarcoma. Porto et al.[5] reported an overall incidence of hematogenous brain metastasis in 0.9% of children with solid tumors in which none was from a case of Ewing's sarcoma.
Since the outcomes of metastatic Ewing's sarcoma are poor, early diagnosis is crucial with a multimodal approach[8] for the treatment of primary tumor. Herein, we report a case of Ewing's sarcoma of the extremity metastasizing to the sellar and suprasellar region involving the left orbital apex along with bilateral lung, abdominal, and left femoral head metastases.
CASE REPORT
A 13-year-old female with a history of below the knee amputation 3 months prior for Ewing's sarcoma [Figure 1], presented to her primary care physician, with left hemicranial headache and rapidly progressive diminution of vision in the left eye along with drooping of eyelid, binocular diplopia, and difficulty in moving the eye for 2 weeks. She also gave a history of single episode of projectile vomiting associated with nasal bleed for which a noncontrast computed tomography (NCCT) of the head was performed. This NCCT revealed a large heterogeneously enhancing mass lesion approximately 34 mm × 26 mm in the sellar and suprasellar region compressing optic chiasma suggestive of pituitary macroadenoma. The patient was referred to our institution for neurosurgical intervention of this pituitary macroadenoma diagnosed at the outside institution. However, the patient did not present until 2 weeks later.
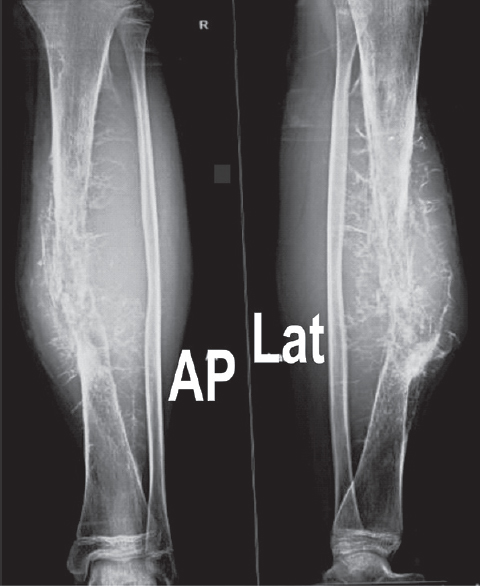
- Anteroposterior and lateral radiograph of the right leg showing lytic lesion with large soft-tissue component and hair-on-end periosteal reaction in mid-diaphysis of tibia
Clinical evaluation at this time revealed no additional ocular or neurological complaints. Her family history and previous history for any genetic disorders were also unremarkable. In the right eye, best-corrected Snellen's visual acuity was 6/6 with no obvious ocular abnormality. In the left eye, there was no light perception, and there was axial proptosis (2 mm), total ptosis, total ophthalmoplegia [Figure 2a and b], total afferent pupillary defect with total optic atrophy, and loss of corneal reflex. Visual-field examination revealed right temporal hemianopia. The remainder of the neurologic examination was normal.
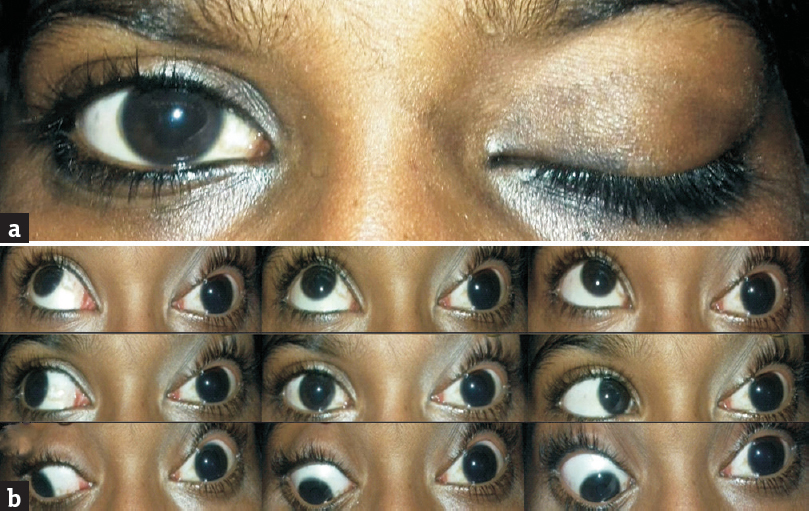
- Left eye. (a) Total ptosis, (b) total ophthalmoplegia
Otorhinolaryngologic evaluation revealed an irregular mass in the right nasopharynx arising from its posterosuperior wall, and an irregular mass in the left sphenoethmoid recess extending into the left nasopharynx. The patient's blood analysis revealed slightly low levels of pituitary hormones (Prolactin 2.7 ng/ml [normal 2.8–29.2 ng/ml], thyroid stimulating hormone 0.27 μIU/mL [normal 0.35–0.50 μIU/mL]) with increased lactate dehydrogenase (884.54 U/L [normal < 247 U/L]) and alkaline phosphatase (456.3 U/L [normal 50–162 U/L]). Magnetic resonance imaging brain at this time [Figure 3a] demonstrated an extensive hyperintense mass lesion centered over sella, engulfing the pituitary gland, compressing the optic chiasma and completely occupying the left cavernous sinus, attenuating the left internal carotid artery (ICA), and abutting the right cavernous sinus and right ICA. The mass was further extending to the sphenoid and posterior ethmoid sinuses, with a small extension to the left orbital apex partially encasing the left optic nerve. The mass had destroyed the clivus and had extended inferiorly into the left nasopharynx and posteriorly into the prepontine cistern. The left Meckel's cave was also involved. Whole-body imaging revealed multifocal irregular heterogeneously enhancing soft-tissue lesions of variable size suggestive of lung and mediastinal metastases [Figure 3b and c]. We also found a lesion with similar enhancement (32 mm × 28 mm × 26 mm) in the right cardiophrenic angle encasing and displacing the posthepatic inferior vena cava anteriorly, abutting the right atrium and distal thoracic esophagus. A few enhancing lymph nodes in both axillae and in the mesentery and a focal lytic defect of the left femoral head was also found [Figure 3d].
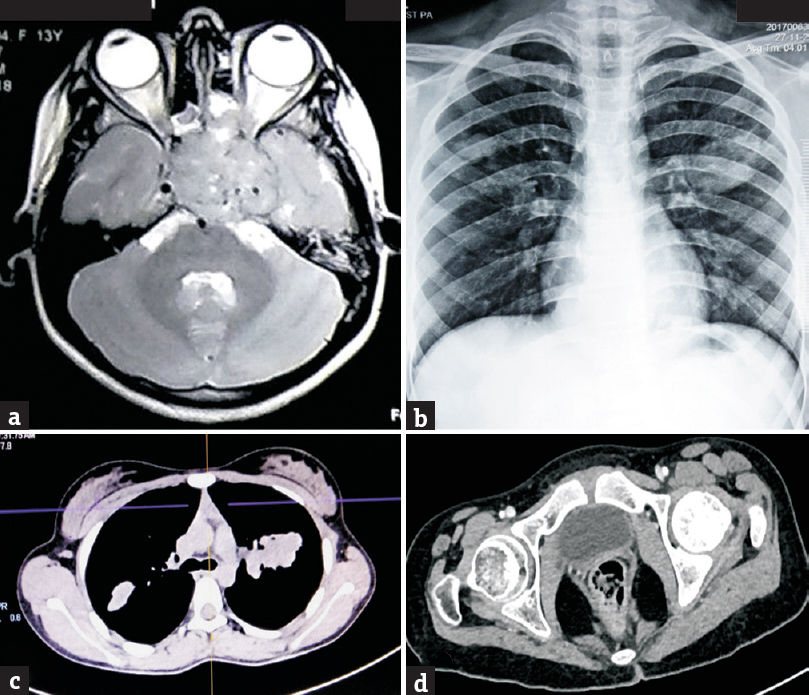
- (a) Magnetic resonance imaging brain showing large mass based on sellar/suprasellar region extending to the left orbital apex, ethmoidal air cells, and cavernous sinus. (b) Chest X-ray showing well-defined opacities in bilateral mid-zones. (c) Computed tomography chest showing irregular soft-tissue lesions in lungs and mediastinum. (d) Computed tomography pelvis showing lytic lesion in the left femoral head
Histopathology of punch biopsy from the nasopharyngeal extension of the tumor revealed nests of small round blue cells in the subepithelium suggestive of a small round-cell carcinoma. Strong CD99 positivity, borderline synaptophysin immunoreactivity, and chromogranin nonreactivity supported the diagnosis of Ewing's Sarcoma [Figure 4a and b].
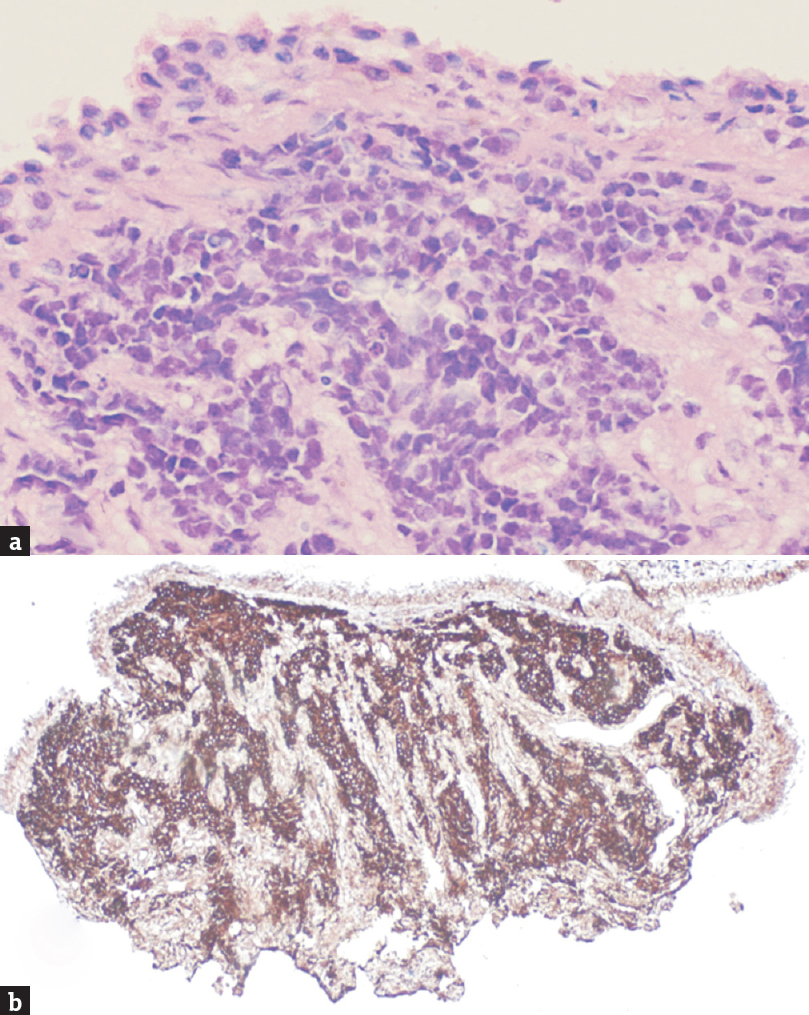
- (a) H and E-stained section of nasopharyngeal punch biopsy specimen showing nests of small round blue cells, (b) Strong CD99 immunoreactivity
As per discussion in our Institutional tumor board, we planned neoadjuvant chemoreduction followed by surgery for this Stage IVb tumor (T3N1M1b) which was classified as unfavorable intermediate risk group of metastatic Ewing's sarcoma as per Ladenstein et al.[91011] On subsequent follow-up after two cycles of chemotherapy with vincristine, doxorubicin, cyclophosphamide intervened with ifosfamide, and etoposide, the patient developed loss of vision in the right eye and dysphagia, due to expansion of the CNS and mediastinal masses. In view of this, emergency decompression was planned; however, patients relatives refused surgery due to expected poor prognosis. The patient finally died of cardiorespiratory failure.
DISCUSSION
In pediatric patients, majority of brain tumors including sellar masses are primary, metastatic involvement accounting for only 1%–10%.[3] Sites of primary for the CNS metastatic tumors in children when compared to adults, are vastly different, with kidney, adrenals, bone, and soft tissue being the most common.[312]
Ewing's sarcomas, rare neoplasia of childhood, typically presenting with an osseous primary, mostly metastasize to lung, bone, and bone marrow[1314] and have a low incidence of CNS metastasis. In the majority of cases, CNS metastasis is the result of direct extension of bony metastasis in proximity to the brain tissue, while hematogenous brain metastases occur in less than 1.8% of the cases.[4715] Up to 80%–90% have subclinical metastasis at the time of presentation; however, the upfront metastatic disease is found in <25% of patients at the time of diagnosis.[1016]
Deopujari et al.[17] in their review of different pediatric suprasellar tumors have remarked that sellar and suprasellar tumors form a separate entity as far as incidence, histology, and responsiveness to therapy is concerned and metastatic tumors such as Ewing's/PNET are rare to this region.[317181920] Ewing's presenting as a sellar mass is a diagnostic and therapeutic challenge as in our case.[18] In our patient, with a history of resected Ewing's sarcoma of tibia, NCCT head at the initial presentation of the patient to her primary physician revealed an intracranial sellar lesion without any apparent osseous involvement of the calvarium mimicking a pituitary adenoma. However, repeat neuroimaging was done at her presentation to our institution 2 weeks later, which showed significant interval growth with differentials shifting towards a metastatic Ewing's sarcoma, and this was supported by immunohistochemical studies on the biopsy specimen. This corroborates with the findings of Weins and Hattab,[3] who in their retrospective review of 20 years of pathological spectrum of CNS metastases in 1135 cases in pediatric population, found only a single case of metastatic sellar/suprasellar involvement of Ewing's sarcoma.
Among the factors altering the prognosis significantly in Ewing's sarcoma, serum LDH levels have been defined as an effective biomarker with high serum levels having low overall survival.[21] In addition, a high probability of subclinical metastasis and a decrease in survival independent of the site of primary tumor has been documented in cases with gross soft-tissue extensions at the primary site of tumor.[22] Our patient had an aggressive primary tumor which led to multiple distant metastases and a poor response to therapy and survival. This may have been due to the large soft-tissue component at the primary site (tibia) as evident on her initial radiograph of the right leg, and her high LDH levels.[2122]
Our case is hence unusual in its presentation as a rare case of sellar metastasis of Ewing's sarcoma in pediatric age group which was initially indistinguishable radiologically from pituitary macroadenoma[2023] and highlights the need of appropriate medical oncological workup at the time of initial presentation/surgery. This also emphasizes that by the time CNS involvement is diagnosed in these patients, they have had multiple metastasis and a poor prognosis for survival despite aggressive therapy.[1016] Thus, a multimodal approach with chemotherapy, surgery, and radiotherapy is recommended for all primaries irrespective of their extent and site to enhance the life expectancy in this young patient population of Ewing's Sarcoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342-52.
- [Google Scholar]
- Ewing's sarcoma of the orbit with intracranial extension: A rare cause of unilateral proptosis. J Pediatr Neurosci. 2011;6:36-9.
- [Google Scholar]
- The pathological spectrum of solid CNS metastases in the pediatric population. J Neurosurg Pediatr. 2014;14:129-35.
- [Google Scholar]
- CNS metastases from bone and soft tissue sarcomas in children, adolescents, and young adults: Are they really so rare? Biomed Res Int 2017 2017 1456473
- [Google Scholar]
- The role of magnetic resonance imaging in children with hematogenous brain metastases from primary solid tumors. Pediatr Hematol Oncol. 2010;27:103-11.
- [Google Scholar]
- Metastatic Ewing's sarcoma to the brain: Case report and review of treatment. Surg Neurol. 1989;31:234-8.
- [Google Scholar]
- Brain metastasis in bone and soft tissue cancers: A review of incidence, interventions, and outcomes. Sarcoma 2014 2014 475175
- [Google Scholar]
- Primary orbital Ewing's sarcoma presenting with local recurrence to maxillary sinus shortly after tumor resection. J Surg Case Rep 2015 2015 pii: rjv070
- [Google Scholar]
- Primary disseminated multifocal Ewing sarcoma: Results of the euro-EWING 99 trial. J Clin Oncol. 2010;28:3284-91.
- [Google Scholar]
- Metastatic Ewing's sarcoma: Revisiting the “Evidence on the fence”. Indian J Med Paediatr Oncol. 2017;38:173-81.
- [Google Scholar]
- Ewing sarcoma: Current management and future approaches through collaboration. J Clin Oncol. 2015;33:3036-46.
- [Google Scholar]
- Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865-72.
- [Google Scholar]
- Intracranial dural metastasis of Ewing's sarcoma: A case report. Korean J Radiol. 2008;9:76-9.
- [Google Scholar]
- The frequency of isolated CNS involvement in Ewing's sarcoma. Cancer. 1982;49:2404-9.
- [Google Scholar]
- Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: A long-term follow-up of the first intergroup study. J Clin Oncol. 1990;8:1664-74.
- [Google Scholar]
- Rare presentation of Ewing sarcoma metastasis to the sella and suprasellar cistern. Clin Imaging. 2017;41:73-7.
- [Google Scholar]
- Diagnosis, treatment, and outcome of pituitary tumors and other abnormal intrasellar masses. Retrospective analysis of 353 patients. Medicine (Baltimore). 1999;78:236-69.
- [Google Scholar]
- Prognostic significance of serum lactate dehydrogenase levels in Ewing's sarcoma: A meta-analysis. Mol Clin Oncol. 2016;5:832-8.
- [Google Scholar]
- The prognostic significance of soft tissue extension in Ewing's sarcoma. Cancer. 1983;51:913-7.
- [Google Scholar]
- Nonadenomatous tumors of the pituitary and sella turcica. Top Magn Reson Imaging. 2005;16:289-99.
- [Google Scholar]






