Translate this page into:
Pure arterial malformation: Reports of two rare cases
*Corresponding author: Manoj Kumar Nayak, Department of Radiodiagnosis, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. tuna.manoj@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vats A, Naik S, Bhoi S, Mishra B, Sahoo B, Nayak M. Pure arterial malformation: Reports of two rare cases. J Neurosci Rural Pract. 2024;15:515-8. doi: 10.25259/JNRP_55_2024
Dear Editor,
Intracranial pure arterial malformation (PAM) is a rare condition defined by the presence of tortuous and dilated arteries forming an arterial mass with a coil-like appearance, devoid of any venous component, as described by McLaughlin et al.[1] The reported incidence of PAM is low with only a limited number of cases documented in the literature. Typically, patients with PAM are either asymptomatic or exhibit non-specific complaints such as headaches or seizures, and these malformations are often discovered incidentally during imaging studies (computed tomography [CT]/magnetic resonance imaging [MRI]). Diagnosing PAM necessitates CT/magnetic resonance angiography (MRA) and digital subtraction angiography (DSA) with 3D acquisitions. While no definitive management strategy has been established for PAM, conservative management is commonly employed based on existing literature.[2] In this series, we presented two cases of PAM affecting different age groups and brain territories, both of which were managed conservatively. Further, research and clinical observation are needed to develop more specific treatment approaches for this condition.
First patient is a 16-year-old female, a known case of seizure disorder since five years, presented for further evaluation of uncontrolled seizures. Despite being compliant with medications, she continued to have two to three episodes of seizures per day and had suffered three instances of status epilepticus in the past five years. There was no family history of seizures, and examination revealed no weakness or sensory loss. The MRI of the brain showed focal thickening of the left hippocampus and amygdala with T2/fluid-attenuated inversion recovery hyperintensities without restriction or blooming in the left medial temporal lobe, hippocampus, and amygdala. Few focal T2 flow voids are seen in the left anteromedial temporal region. An MRA-time of flight showed non-visualization of the left intracranial internal carotid artery (ICA). The basilar artery was dilated with a maximum luminal diameter of 5.6 mm. The left posterior cerebral artery (PCA) and posterior communicating artery (PCoM) were dilated with tortuous, coil-like vessels in the left anteromedial temporal region without any venous communication [Figures 1a-d]. No evidence of associated aneurysm or calcification was seen. The DSA showed a faint visualization of the left ICA. The left vertebral artery run showed dilated left P2 PCA and PCoM with multiple coil-like arteries without early draining veins. These findings were suggestive of PAM [Figure 1e-h]. The left ICA was replaced with an ascending pharyngeal artery [Figure 1g]. Anti-seizure medications were optimized, and she achieved good seizure control. At one-year follow-up, she was seizure free and had no other neurological deficits.
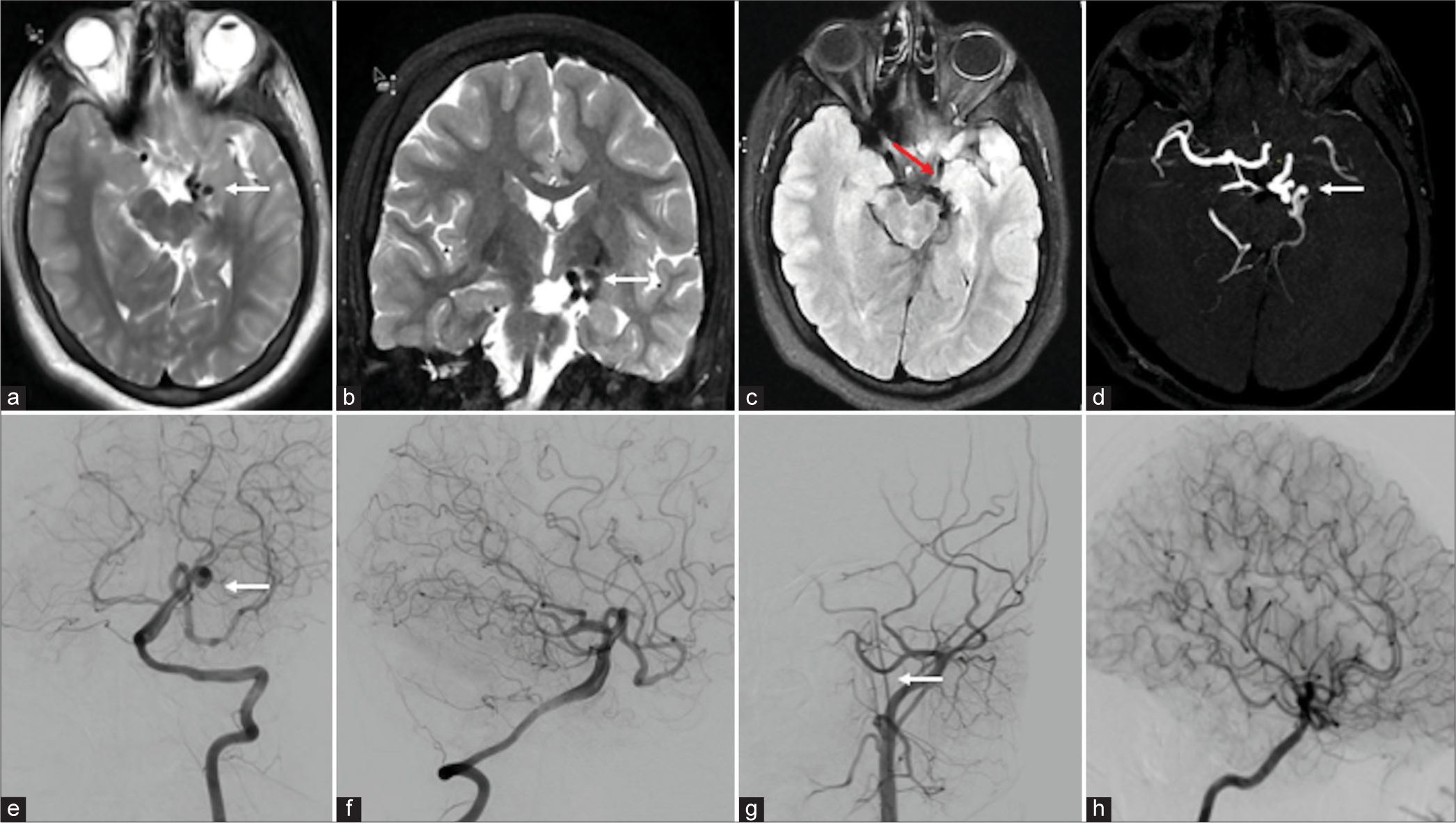
- (a-d) Magnetic resonance imaging (MRI) and digital subtraction imaging showed the imaging features of pure arterial malformation (PAM) MRI image: (a) Axial T2, (b) coronal T2, and (c) axial fluid-attenuated inversion recovery showed abnormal coiled-like vessels in the left side of the suprasellar cistern (white arrows) with dysplastic left hippocampus and medial temporal lobe (red arrow). (d) Time-of-flight circle of Willis showed coiled-like vessels in the left posterior cerebral artery (PCA) without abnormal veins (white arrow). (e-h) Digital subtraction angiography image: (e and f) Vertebral artery runs showed coiled-like vessels in the left PCA without abnormal draining vein (white arrow). (g) The left common carotid artery run showed non-visualization of the left internal carotid artery (ICA) from bifurcation and continued as an ascending pharyngeal artery (white arrow). (h) The right ICA run showed multiple leptomeningeal collaterals in the right cerebral parenchyma.
The second patient is a 48-year-old male, without any previous comorbidities, presented with intermittent headache, once every month without aura, forgetting names, and irrelevant talk (transient Wernicke aphasia), recovered fully in four days. An MRI of the brain showed multiple dilated vessels near the right supraclinoid ICA and right pericallosal artery without early draining veins along with pachygyria in the underlying cingulate cortex [Figures 2a-c]. The DSA showed a dilated arterial system in the right ICA and right pericallosal artery without abnormal early draining veins suggestive of PAM [Figures 2d and e]. There was also non-visualization of the left ICA from its origin and appeared replaced with an ascending pharyngeal artery [Figure 2f]. The patient was managed conservatively and symptomatically improved at four-month follow-up.
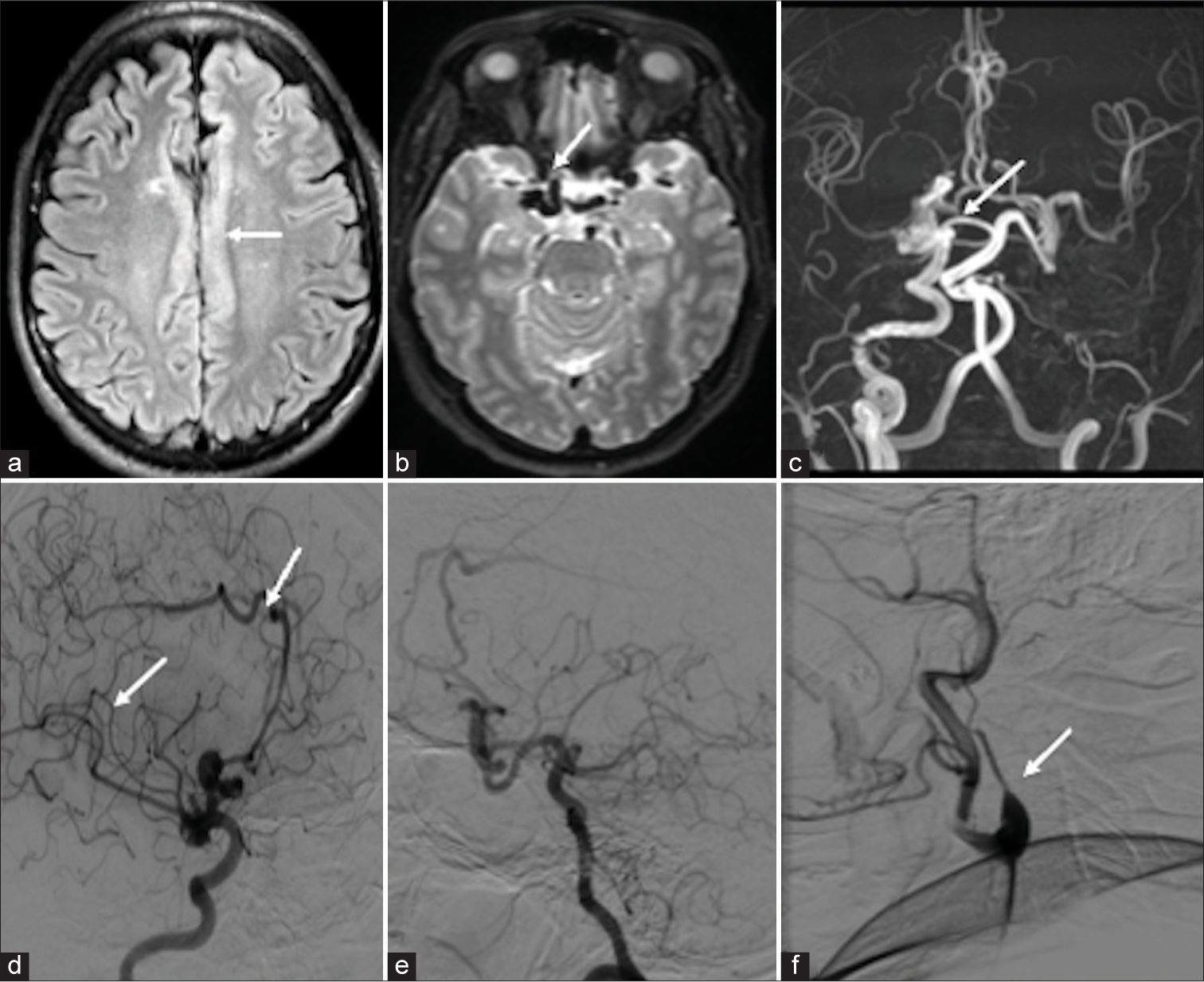
- (a-c) Magnetic resonance imaging (MRI) digital subtraction imaging showed the imaging features of pure arterial malformation (PAM) MRI image: (a) Axial fluid-attenuated inversion recovery showed pachygyria over the cingulate cortex (white arrow), (b) axial T2 image showed multiple abnormal dilated vessels in the right supraclinoid internal carotid artery (ICA) (white arrow), and (c) time-of-flight circle of Willis showed dilated right supraclinoid ICA and right pericallosal artery without abnormal veins and non-visualization of the left ICA (white arrow). (d-f) Digital subtraction angiography image: (d) Right common carotid artery (CCA) lateral showed abnormally dilated arteries in the right supraclinoid ICA, pericallosal artery with aneurysm, without abnormal draining vein (white arrow). (e) The right vertebral artery showed multiple collaterals and filling of the left middle cerebral artery through the left posterior communicating artery. (f) The left CCA run showed non-visualization of the left ICA from bifurcation and continued as ascending pharyngeal artery (white arrow).
Intracranial PAM is defined as the presence of tortuous and dilated arteries, which form an arterial mass, giving a coil-like appearance, without having any venous component within it. There may be the presence of associated aneurysms or calcifications.[3] Among the very few cases described in the literature, most of the patients presented with non-specific neurologic symptoms, the most common being headache and seizures, few patients presented with subarachnoid hemorrhage and infarcts.[4]
For diagnosis of PAM, a CT/MRA is required, and confirmation is done by DSA. On these imaging modalities, these lesions appear as a focal coil-like mass formed by dilatation and tortuosity of intracranial arteries. There is an absence of any venous component or drainage, which helps to differentiate this malformation from other intracranial vascular malformations, like arteriovenous malformation or arteriovenous fistula. The most commonly involved arteries in PAM are supraclinoid ICA, PCoM, anterior cerebral artery (ACA), and M1 middle cerebral artery (MCA).[5] Another close differential of PAM is developmental arterial anomalies, which usually involve smaller intracranial arteries and can be associated with cortical dysplasia.[6] During normal brain development, arterial growth follows the pattern of cortical development, and neural crest cells help in the development of tunica media of anterior circulation and neuronal migration.[7] Hence, arterial dysplasia can be associated with underlying cortical malformation like hippocampal dysplasia causing seizures. Another possible explanation for arterial dysplasia in PAM could be due to a compensatory overflow in the abnormal vessels in the absence of the ICA continuing as an ascending pharyngeal artery as a causal relationship with PAM. In our first case, posterior circulation arteries (PCA and PCoM) were affected with associated focal hippocampal and amygdala dysplasia. Other differential diagnoses of this disease may be arterial dissection and dolichoectasia. Hematoma on the vascular wall or double cavity sign helps in differentiating dissection from PAM on imaging. However, some theories suggested that there is a causal relationship between PAM and dissection.[3] Dolichoectasia affects the large caliber vessels such as the vertebrobasilar system and ICAs, predominantly in older populations whereas PAM affects the small caliber intracranial vessels.[2]
The etiopathogenesis of this disease is still unclear, and potential etiologies can be (1) a chronic healed dissection; (2) an insult such as a somatic mutation or viral infection causing segmental arterial vulnerability; or (3) a congenital insult causing arterial dysplasia[2] Araki et al. reported a case of PAM on the right ACA, MCA, and PCA on DSA and CT images with the right hemimegalencephaly on a 23-year-old suggesting congenital etiology.[5] The PAMs were associated with cortical dysplasia or white matter abnormality in the region of the affected vessels in five cases to suggest the congenital nature of the disease.[4] Mackel et al. reported a case of dilated anterior communicating artery associated with other cranial neurocristopathies such as hypoplastic ICA, transsphenoidal encephalocele, ectopic, duplicated pituitary gland, and persistent craniopharyngeal canal attributed to neural crest failure.[8] We also support this hypothesis, as tunica media of vessels in anterior circulation including PCAs develop neural crest disease. Neural crest cells help in neurogenesis and neuronal migration, which explains the nature of this disease and its association with intracranial disease.
The management of PAM is not yet well understood. Conservative management with serial imaging follow-up, cessation of smoking, and controlling of hypertension is the approach of treatment at present.[2] Those patients presented with aneurysmal subarachnoid hemorrhage can be treated surgically or with endovascular treatment. The perfusion of the affected hemisphere in symptomatic patients can be increased by wrapping the MCA by muscle graft combined with extra-intracranial bypass by Hanakita et al.[9] or ICA-radial artery-M2 bypass, superficial temporal artery-P2 bypass.[4] Post-operative angiogram showed the disappearance and regression of these malformations at six-month follow-up with a reduction of bleeding risk in the future. In our case, the patient had a long history of seizures from the past five years and was receiving anti-epileptics with which she had a partial response with intermittent ictal episodes. However, since there was no associated aneurysmal dilatation within the malformation, the patient was medically managed, and epilepsy was controlled with medications.
The PAM is a rare cerebrovascular anomaly with limited documented cases. Recognizable by its distinct arterial features, management options range from conservative to surgical interventions based on symptomatology. This report serves as a valuable resource for clinicians and radiologists, aiding in the diagnosis and treatment of this uncommon condition. Continued research and collaboration are crucial for advancing our understanding and improving patient care for PAM.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Pure arterial malformation of the posterior cerebral artery: Importance of its recognition: Case report. J Neurosurg. 2013;119:655-60.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of pure arterial malformation: Three case reports and literature review. Medicine (Baltimore). 2020;99:e20229.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral revascularization for the management of symptomatic pure arterial malformations. Front Neurol. 2021;12:755312.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital hemicerebral arterial ectasia complicating unilateral megalencephaly. Br J Radiol. 1987;60:395-400.
- [CrossRef] [PubMed] [Google Scholar]
- Arterial vascular abnormality accompanying cerebral cortical dysplasia. AJNR Am J Neuroradiol. 1997;18:144-6.
- [Google Scholar]
- Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N Am. 2010;21:399-426.
- [CrossRef] [PubMed] [Google Scholar]
- Neural crest cell failure as embryogenesis for fusiform aneurysm of the anterior communicating artery: Case report and review of the literature. World Neurosurg. 2019;129:232-6.
- [CrossRef] [PubMed] [Google Scholar]
- Surgically treated cerebral arterial ectasia with so-called moyamoya vessels. Neurosurgery. 1986;19:271-3.
- [CrossRef] [PubMed] [Google Scholar]





