Translate this page into:
Pseudosubarachnoid hemorrhage sign in a patient with tetralogy of Fallot
*Corresponding author: Ramesh S. Doddamani, Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi, Delhi, India. drsdramesh@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Goyal S, Jain A, Doddamani RS, Siddharthan D, Singh M, Chandra PS. Pseudosubarachnoid hemorrhage sign in a patient with tetralogy of Fallot. J Neurosci Rural Pract. 2024;15:503-6. doi: 10.25259/JNRP_643_2023
Abstract
Subarachnoid hemorrhage (SAH) constitutes a common neurosurgical emergency. Non-contrast computerized tomography (NCCT) head has very high specificity and sensitivity in detecting SAH. However, in certain conditions, the computed tomography (CT) may be falsely positive, termed as pseudo-SAH. We hereby report a rare case of pseudo-SAH in a patient with tetralogy of Fallot and discuss the possible etiopathogenesis and the pertinent literature. We present here a case of a 15-year-old male patient, who was diagnosed with tetralogy of Fallot (TOF) physiology. He presented to the cardiology outpatient clinic with complaints of dyspnea on exertion, headache, and drowsiness. The NCCT head revealed diffuse hyperdensity in the basal cisterns and sulcal spaces, which were suggestive of diffuse SAH. However, in the setting of negative CT and magnetic resonance angiography along with high hematocrit levels (84%), diagnosis of pseudo-SAH was considered. The patient was appropriately managed with beta-blockers, intravenous fluids, and serial phlebotomies. The patient underwent a palliative shunt connecting the ascending aorta to the main pulmonary artery (central shunt), following which his saturation improved, and hematocrit decreased (40%) leading to significant symptomatic improvement. Knowledge of pseudo-SAH signs is essential for the treating physician and plays an important part in the management of this patient population.
Keywords
Pseudosubarachnoid hemorrhage
Tetralogy of Fallot
Subarachnoid hemorrhage
Cyanotic heart disease
INTRODUCTION
Subarachnoid hemorrhage (SAH) constitutes a common neurosurgical emergency. Non-contrast computerized tomography (NCCT) is routinely performed as a preliminary investigation for patients with acute-onset severe headache or altered sensorium. Although SAH is primarily detected on the NCCT head as a part of initial evaluation, computed tomography angiography (CTA) or digital subtraction angiography (DSA) of the cerebral vessels is further needed to rule out vascular pathologies such as aneurysms and arteriovenous malformations requiring emergency interventions.
The NCCT head has very high specificity and sensitivity in detecting SAH. However, in certain conditions, computed tomography (CT) may reveal SAH falsely in the absence of blood in the subarachnoid space, termed as pseudo-SAH. Pseudo-SAH can be caused by the increase in hyper-attenuating substances in sulcal and basal cisterns either in the intravascular spaces (stagnation of blood in disease with high hematocrits such as polycythemia vera and cyanotic congenital heart diseases) or in extravascular spaces (purulent meningitis, meningeal leukemia, and gliomatosis). We hereby report a case of pseudo-SAH in a patient with tetralogy of Fallot and discuss the possible etiopathogenesis and the relevant literature.
CASE REPORT
We present here a case of a 15-year-old boy, who was diagnosed with tetralogy of Fallot (TOF). He presented to the cardiology outpatient clinic with complaints of breathlessness on exertion, New York Heart Association (NYHA) class 2 symptoms since childhood, moderate headache and drowsiness for two days. Headache was described as continuous and of moderate intensity. There was no history suggestive of sudden-onset headache. Examination revealed facial cyanosis and grade IV clubbing of fingers indicating chronic hypoxemia [Figure 1]. The patient was drowsy with a Glasgow coma scale (GCS) of 14/15 (E3V4M6), maintaining oxygen saturation of 67% on the pulse oximeter. Left-sided ptosis was noted with restricted eyeball movements and dilated pupil compared to the right eye, suggestive of left third nerve palsy, as the patient was able to abduct the left eyeball.
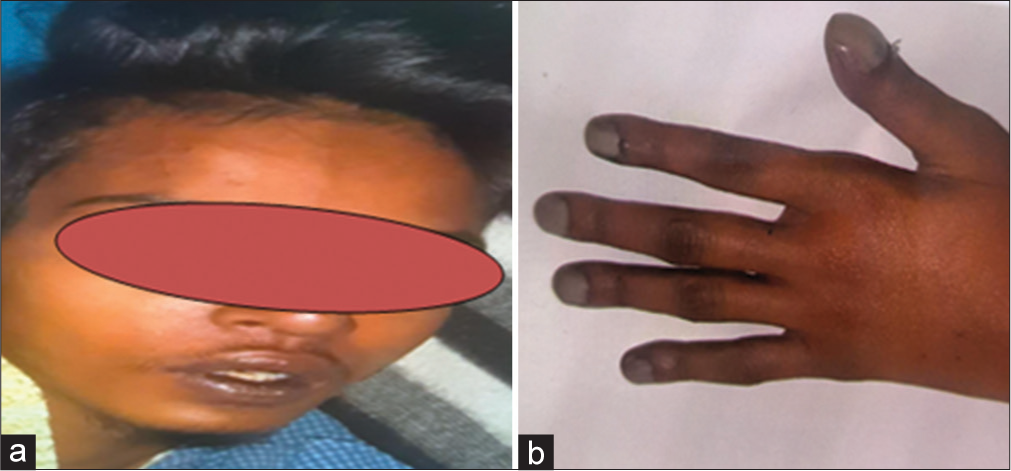
- A 16-year-old male was admitted in a drowsy state (a) Profound cyanotic lips due to chronic hypoxemia secondary to tetralogy of Fallot can be noticed. (b) Grade 4 clubbing of fingers due to chronic hypoxemia.
The NCCT head revealed diffuse hyperdensity in the basal cisterns and sulcal spaces suggestive of diffuse SAH [Figure 2]. His laboratory investigations revealed hemoglobin of 26 g/dL with a hematocrit of 84%, suggesting a compensatory mechanism for chronic hypoxemia. A CTA of the brain did not show any evidence of aneurysm and vascular malformations; however, evidence of dilated vascular channels was noted throughout the brain [Figure 2].
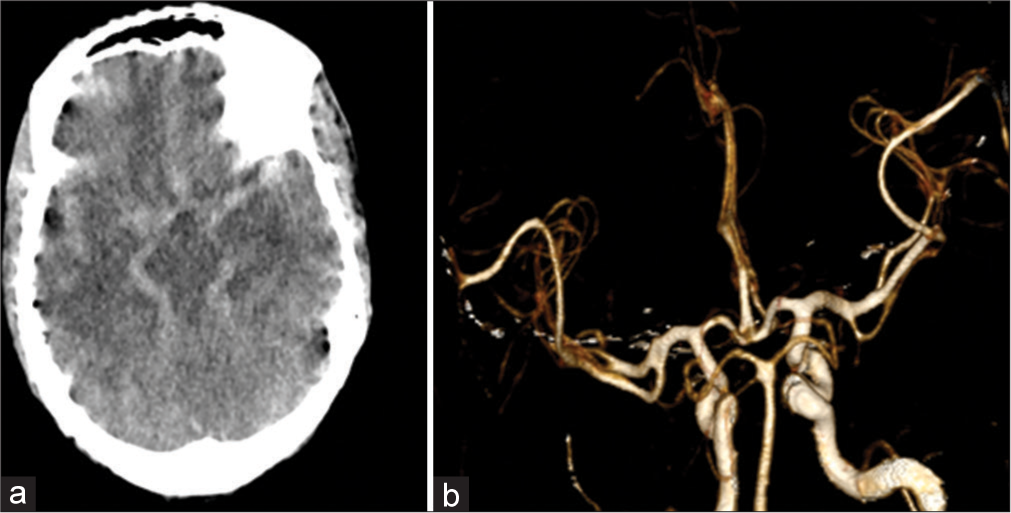
- (a) Axial non-contrast computerized tomography head showing diffuse hyperintensity in basal cisterns as well as in the cortical sulcal spaces, appearing as subarachnoid hemorrhage. (b) 3D Recon volume-rendering technique images of computed tomography angiogram showed normal vessels.
As the diagnosis of SAH was strongly debated, magnetic resonance imaging (MRI) brain for further clarification was performed [Figure 3]. An MRI brain revealed diffuse blooming in susceptibility weighted imaging (SWI) sequence without obvious fluid-attenuated inversion recovery (FLAIR) hyperintensities in the sulcal spaces of cerebral and cerebellar hemispheres bilaterally. Magnetic resonance angiography (MRA) time of flight (TOF) sequence and contrast-enhanced MRA revealed normal intracranial vessels along with the marked proliferation of vascular channels throughout the brain. In view of the abovementioned MRI brain findings, the possibility of pseudo-SAH in the differential diagnosis was considered.
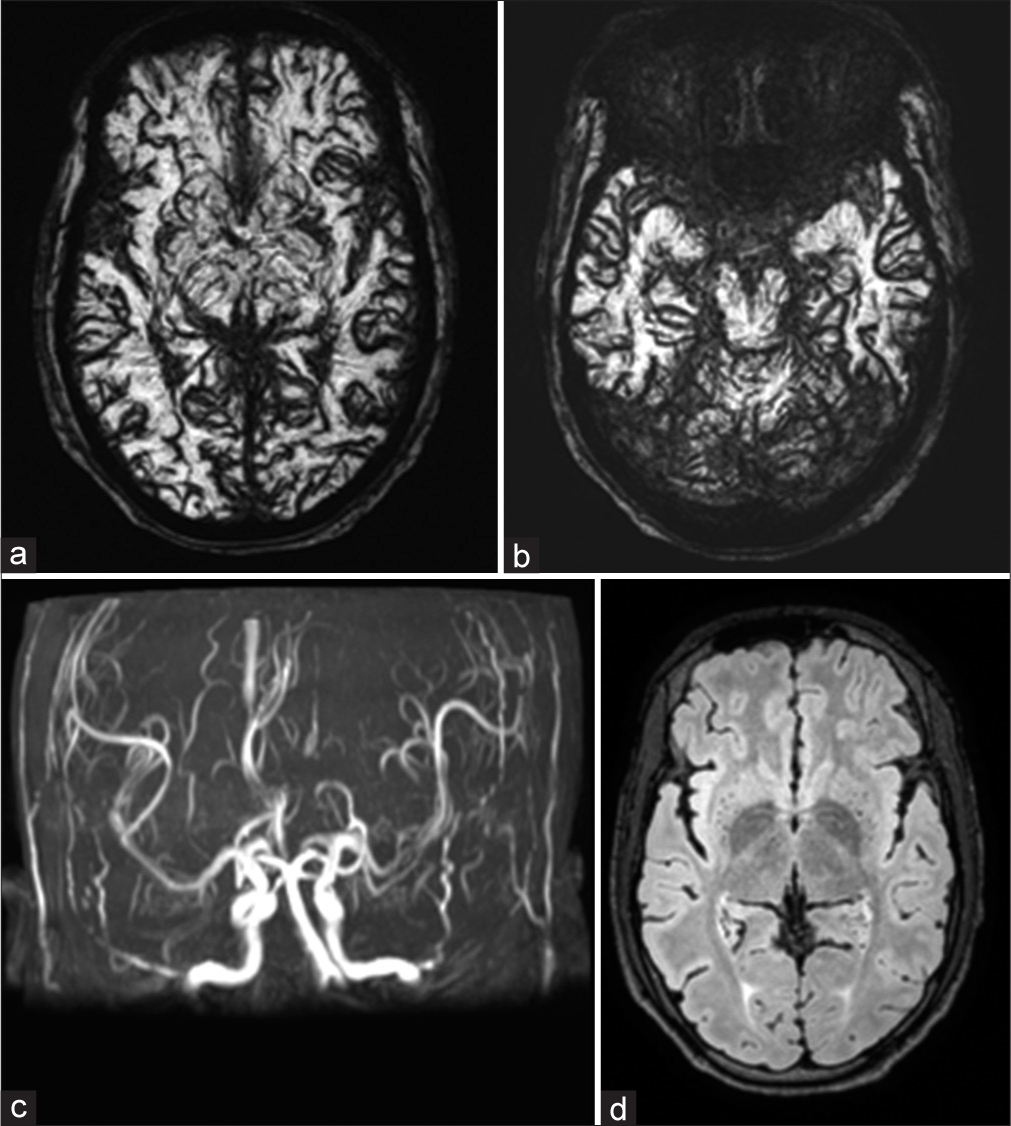
- (a and b) Susceptibility-weighted imaging sequence of magnetic resonance imaging brain shows diffuse blooming in the sulcal spaces and basal cisterns of bilateral cerebral hemispheres and cerebellar folia. (c) MIP magnetic resonance angiography (MRA) TOF and contrast-enhanced MRA sequences revealed normal intracranial vessels without any evidence of aneurysm or arteriovenous malformations. (d) Fluid-attenuated inversion recovery sequence revealed the absence of hyperintensities in sulcal spaces and basal cisterns, respectively, ruling out the presence of subarachnoid hemorrhage.
The patient was appropriately managed with beta-blockers, intravenous fluids, and serial phlebotomies. The hematocrit values decreased to 60% following which an improvement in the patient’s sensorium was observed (GCS-15/15).
He was transferred to the cardiothoracic surgery department and subsequently underwent a palliative shunt connecting the ascending aorta to the main pulmonary artery (central shunt) (central shunt is a conduit placed between the ascending aorta to the pulmonary artery to increase the volume of blood flow in the pulmonary artery and hence the lungs, thereby improving the saturation and decreasing the hypoxia). The patient showed a dramatic improvement in blood oxygen saturation, and a further drop in hematocrit (50%) was noted and an improvement in sensorium. Repeat NCCT head after three weeks of the initial CT was performed as a part of a routine evaluation to look for the status of the pseudo-SAH [Figure 4]. Resolution of hyperdensities in the basal cisterns and the convexity sulci was noted. Incidentally, a small quantity of blood in the fourth ventricle and lateral ventricles was also noted [Figures 4a and b]. This could be attributed to the use of anticoagulants during central shunt surgery. The patient did not develop any features of hydrocephalus, as he was closely observed in the ICU. Resolution of hyperintensities in the basal cisterns and the sulcal spaces in congruence with a reduction in the hematocrit values further proved our diagnosis of pseudo-SAH. On follow-up, three weeks following the cardiac surgery, the patient’s ptosis had resolved along with improvement in the eyeball movements noted.
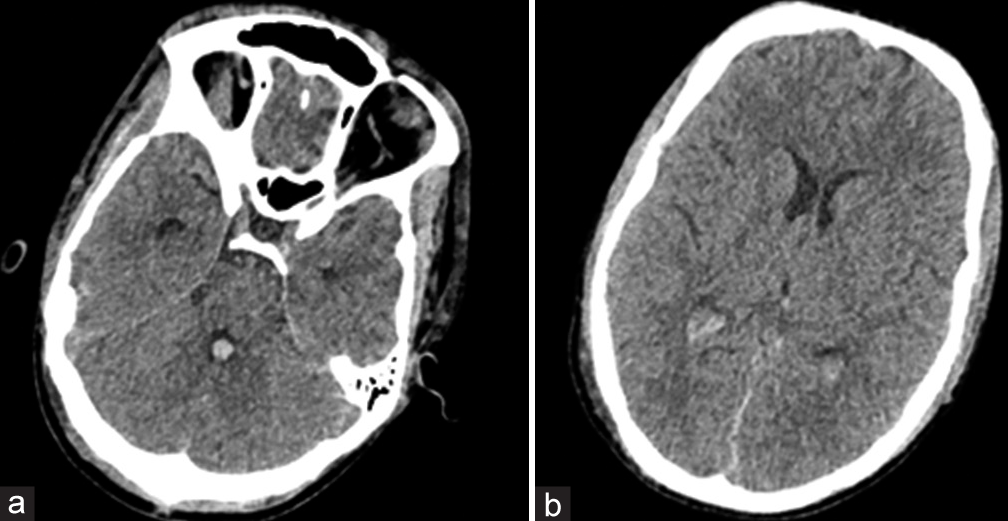
- (a) Repeat non-contrast computerized tomography head after central shunt depicted resolution of hyperdensities in the basal cisterns and the presence of a small amount of intraventricular hemorrhage (IVH) in the fourth ventricle. (b) A small amount of IVH also was noted in the bilateral occipital horns of the lateral ventricle.
DISCUSSION
“Pseudo-SAH” refers to hyperattenuation of the sulci and basal cisterns in the NCCT head deceptively appearing as SAH in the absence of actual blood in the cisternal spaces.[1]
Pseudo-SAH appearance results owing to an increase in the difference of attenuation of basal cisterns and sulcal spaces compared to the cerebral parenchyma. This can be caused by the increase in hyper-attenuating substances in sulcal and basal cisterns either in the intravascular spaces (stagnation of blood in diseases with high hematocrits such as polycythemia vera[2] and cyanotic congenital heart diseases[3]) or in extravascular spaces (purulent meningitis,[4] meningeal leukemia,[5] and gliomatosis[6]). Pseudo-SAH sign can also result from a decrease in attenuation values of cerebral and cerebellar parenchyma as seen in cerebellar infarctions and severe hypoxic or anoxic encephalopathy[7] in post-cardiac arrest survivors. Pseudo-SAH sign has also been reported in patients who received intravenous,[8] intrathecal contrast agents,[9] patient with bilateral chronic subdural hematoma,[10] patients with valproate toxicity,[11] intracranial infections,[12,13] and patients with pulmonary disorders leading to chronic hypercapnia.[14]
An MRI brain is essential in diagnosing this condition where the presence of FLAIR hyperintensities in the sulcal spaces is characteristic of SAH. In the present case, no such hyperintensity was noted, thus ruling out true SAH. The other significant finding was the presence of diffuse blooming in SWI. This finding is again characteristic of dilated vascular channels suggesting evidence of neoangiogenesis due to chronic hypoxemia of the brain.
Our patient had chronic hypoxemia owing to his complex cardiovascular malformation with significant intermixing of blood between the systemic and pulmonary circulations. This resulted in the development of secondary polycythemia due to chronic hypoxemia, progressing to high hematocrit and hyperviscosity-related symptoms. The mechanism of pseudo-SAH in our case could be presumed to be a result of stagnation of blood in the venous channels secondary to polycythemia. A brief overview of various clinical entities that can cause pseudo-SAH sign has been given in Table 1.
| S. No. | Author/year | Patient profile | Presentation/diagnosis | Possible mechanism of pseudo-SAH sign |
|---|---|---|---|---|
| 1. | Patzig et al.[3] 2014 | 37 year/male | Headache in patients with transposition of the great arteries. | Chronic hypoxemia-induced polycythemia leads to stagnated blood in venous channels. |
| 2. | Fretwell et al.[4] 2022 | 64 year/female | Bacterial meningitis with acute ischemic stroke | Exudates in cisternal spaces |
| 3. | Hsieh et al.[5] 2012 | 68 year/male | Meningeal leukemia | Basal cistern hyperdensities |
| 4. | Belsare et al.[6] 2004 | 49 year/male | Gliomatosis cerebri | Brain edema with bilateral occipital lobe infarcts following herniation and compression of the posterior cerebral arteries |
| 5. | You et al.[7] 2008 | 49 year/female | Post-CPR ROSC | Acute hypoxic anoxic encephalopathy coupled with venous engorgement |
| 6. | Honton et al.[8] 2020 | 83 year/female | Coronary artery disease patient who underwent PCI | Intracerebral diffusion of contrast agent |
| 7. | Platt et al.[9] 2020 | 73 year/female | Gadolinium encephalopathy after lumbar epidural steroid injection for lumbar canal stenosis | Iatrogenic intrathecal contrast administration |
| 8. | Shima et al.[10] 2019 | 83 year/female | Bilateral chronic subdural hematoma | Effacement of sulci and basilar cisterns leading to Higher attenuation. |
| 9. | Dadpour et al.[11] 2016 |
18 year/female | Sodium valproate overdose | High serum levels of valproic acid act as a contrast agent in the context of brain edema. |
| 10. | Liu et al.[12] 2021 | 49 year/male | Cerebral cysticercosis | Dense exudates resulting from calcifications of cerebral cysticercosis in basal subarachnoid space |
| 11. | Singh et al.[13] 2021 | 40 year/male | Coccidioidal infection | Basal cistern hyperdensities |
| 12. | Tiwari et al.[14] 2020 | 48 year/male | Headache in a patient with bronchiectasis with pulmonary hypertension | Hypercapnia-induced diffuse cerebral edema coupled with polycythemia |
| 13. | Our patient | 15 year/male | Case of TOF with drowsiness and headache | Chronic hypoxemia-induced polycythemia leads to stagnated blood in venous channels. |
SAH: Subarachnoid hemorrhage, CPR: Cardiopulmonary resuscitation, ROSC: Return of spontaneous circulation, PCI: Percutaneous coronary intervention, TOF: Tetralogy of Fallot’s
CONCLUSION
Knowledge of pseudo-SAH signs is essential for the treating physician, as it is critical in the management of these patients. It aids by avoiding unwarranted invasive investigations such as DSA. In patients with Pseudo-SAH sign in setting of chronic hypoxemia and high hematocrit values, the use of cerebral decongestants such as mannitol and diuretics are contraindicated. These agents can further elevate the hematocrit values leading to deterioration in clinical status by precipitating ischemic strokes due to stagnation of blood.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Pseudo-Subarachnoid hemorrhage: A potential imaging pitfall. Can Assoc Radiol J. 2014;65:225-31.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between hyperhemoglobinemia and pseudosubarachnoid hemorrhage. Clin Imaging. 2020;59:8-12.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo-subarachnoid haemorrhage due to chronic hypoxaemia: Case report and review of the literature. BMC Neurol. 2014;14:219.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo subarachnoid hemorrhage sign in bacterial meningitis in a patient presenting with acute ischemic stroke: A novel radiological clue to rapid diagnosis. Cureus. 2022;14:e25283.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med. 2012;42:e109-11.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo-subarachnoid hemorrhage and cortical visual impairment as the presenting sign of gliomatosis cerebri. Semin Ophthalmol. 2004;19:78-80.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo-subarachnoid hemorrhage. Am J Emerg Med. 2008;26:521.e1-2.
- [CrossRef] [PubMed] [Google Scholar]
- Extended pseudo-subarachnoid hemorrhage post-percutaneous coronary intervention. JACC Case Rep. 2020;2:2394-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo-subarachnoid hemorrhage and gadolinium encephalopathy following lumbar epidural steroid injection. Radiol Case Rep. 2020;15:1935-8.
- [CrossRef] [PubMed] [Google Scholar]
- Bilateral chronic subdural hematoma presenting with pseudo-subarachnoid hemorrhage sign on computed tomography. Asian J Neurosurg. 2019;14:510-2.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo-subarachnoid hemorrhage and optic neuritis in an 18-year-old girl with sodium valproate overdose. J Res Med Sci. 2016;21:43.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral cysticercosis mimicking subarachnoid hemorrhage: A case report. Chin Neurosurg J. 2021;7:39.
- [CrossRef] [PubMed] [Google Scholar]
- Coccidioidal meningitis with multiple aneurysms presenting with pseudo-subarachnoid hemorrhage: Illustrative case. J Neurosurg Case Lessons. 2021;2:CASE21424.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudo-subarachnoid hemorrhage in a patient with acute on chronic respiratory failure. Ann Indian Acad Neurol. 2020;23:706-8.
- [CrossRef] [PubMed] [Google Scholar]






