Translate this page into:
Prognostic factors affecting outcome of multifocal or multicentric glioblastoma: A scoping review
*Corresponding author: Dr. Saikat Das, MD, DNB, M-Tech, MNAMS, PhD, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India. saikat.radiotherapy@aiimsbhopal.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Das S, Mishra RK, Agrawal A. Prognostic factors affecting outcome of multifocal or multicentric glioblastoma: A scoping review. J Neurosci Rural Pract 2023;14:199-209.
Abstract
It has been reported that patients with multiple lesions have shorter overall survival compared to single lesion in glioblastoma (GBM). Number of lesions can profoundly impact the prognosis and treatment outcome in GBM. In view of the advancement of imaging, multiple GBM (mGBM) lesions are increasingly recognized and reported. The scoping review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for systematic review. Database was searched to collect relevant articles based on predefined eligibility criteria. Our observations suggest that multifocal/multicentric GBM has poorer outcome compared to GBM with singular lesion (sGBM). As the factors influencing the prognosis and outcome is poorly understood and there is no consensus in the existing literature, this review is clinically relevant. As patients with single lesion are more likely to undergo gross total excision, it is likely that further adjuvant treatment may be decided by extent of resection. This review will be helpful for design of further prospective randomized studies for optimal management of mGBM.
Keywords
Glioblastoma
Multifocal GBM
Multicentric GBM
Scoping review
Temozolomide
INTRODUCTION
Multiple lesions are reported to be present in glioblastoma (GBM) in 0.5–20% of cases.[1] It has been reported that patients with multiple lesions multicentric or multifocal, (mGBM) have shorter overall survival compared to single lesion.[2] Number of lesions can profoundly impact the prognosis and treatment outcome in GBM.[3] The diagnosis of multiple synchronous lesions or tumor infiltration critically depends on the imaging modality used.[3] Multiple GBM can be classified into multifocal or multicentric lesions.[4] In multicentric lesion, there is no macroscopic or microscopic connection with the primary site and the lesions are usually separated by ≥2 cm or lesion present in contralateral lobe away from the primary lesion.[3,5] In multifocal GBM, the lesion is connected microscopically or through commissural fibers, cerebrospinal fluid, or by local extension. In view of the infiltrative nature of the lesion, maximum safe resection is often not possible for multiple GBM (mGBM). Various studies have shown that distinction of multifocal or multicentricity has little prognostic significance and indeed can be spectrum of same process of disease evolution.[6] Except few, most of the studies reported that mGBM has poorer outcome.[6] Strong correlation of survival is observed with extent of resection (EOR) and Karnofsky performance status (KPS) at presentation.[7] Some studies have reported similar pattern of progression of both unifocal and multifocal GBM[8] though there is difference in molecular biology. For example, EGFR amplifications, CDKN2A/B homozygous deletions, and a CYB5R2 over expression are more frequent in mGBM.[9] There is no consensus on the management of approach of the management of multiple GBM (mGBM) as compared to solitary GBM. Systematic review of the factors determining the outcome of mGBM is relevant and clinically important. Survival pattern is different and most of the patient present in a condition of poor performance status. The objective of the present scoping review is to analyze the factors determining the prognosis and outcome of mGBM.
MATERIALS AND METHODS
The scoping review was done following standard guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses.[10]
Eligibility criteria
Clinical trials including randomized controlled trials (RCTs), quasi-randomized trials, non-randomized studies, and controlled before-and-after studies, case–control studies, matched pair analysis, or studies without a control group reporting outcome of mGBM.
Studies were selected if they met the following criteria:
Studies describing newly diagnosed mGBM (multiple GBM including both multicentric/multifocal) included as part of reported cohort
Pre-operative or post-operative magnetic resonance imaging (MRI) information was available
Studies that had histopathologically confirmed cases
Studies that included patients who underwent surgery (including biopsy) and/or post-operative radiotherapy with or without chemotherapy
Studies that provided information on overall survival.
Case reports, low-grade lesions, and spinal cord involvement were not included. Case series was included.
Information sources and search strategy
Following database was searched to obtain the eligible studies: PubMed, EMBASE, Cochrane Library, SCOPUS, ClinicalTrials.gov, and International Clinical Trials Registry Platform. The reference lists of included studies were searched to identify any other related published articles and additional studies [Table 1]. Two researchers identified suitable studies (SD and AA) in an independent and unbiased manner.
| Search 22.02.2022 | |
|---|---|
| PubMed | |
| Search 1 | 293 |
| Multifocal[All Fields] AND multicentric[All Fields] AND (“glioblastoma”[MeSH Terms] OR “glioblastoma”[All Fields]) AND (“prognosis”[MeSH Terms] OR “prognosis”[All Fields]) | |
| Search 2 | 2443 |
| (Multifocal[All Fields] AND (“glioblastoma”[MeSH Terms] OR “glioblastoma”[All Fields])) AND (“prognosis”[MeSH Terms] OR “prognosis”[All Fields]) | |
| Search 3 | 1118 |
| Multicentric[All Fields] AND (“glioblastoma”[MeSH Terms] OR “glioblastoma”[All Fields]) AND (“prognosis”[MeSH Terms] OR “prognosis”[All Fields]) | |
| SCOPUS | |
| Search 1 | |
| TITLE-ABS-KEY (multicentric AND glioblastoma AND prognosis) | 58 |
| Search 2 | |
| TITLE-ABS-KEY (multifocal AND glioblastoma AND prognosis) | 90 |
| COCHRANE | |
| Multifocal glioblastoma prognosis | 8 |
| EMBASE | |
| Search 1 | |
| TITLE-ABS-KEY (multicentric AND glioblastoma AND prognosis) | 152 |
| Search 2 | |
| TITLE-ABS-KEY (multifocal AND glioblastoma AND prognosis) | 152 |
Selection process
All titles and abstracts retrieved by electronic searching were downloaded to reference manager. Duplicate entries were removed. A minimum of two reviewers (SD and AA) independently screened the search results, rejecting all clearly irrelevant records and categorizing the remaining articles into included studies, excluded studies, ongoing studies, and studies awaiting classification. We obtained the full text of potentially eligible articles. We resolved any disagreements about eligibility by mutual discussion.
Data collection process
We collected the following information in pre-conceived data collection form designed for this review. Each included study was analyzed to collect the following data:
Study title, authors’ name, and year of publication
Country of origin
Total number of patients
Total number of patients with mGBM
Mean age
Gender
KPS/performance status in any other scale
Location of tumors
Treatment details (extent of surgery/radiotherapy/ chemotherapy)
Information on overall survival.
RESULTS
A total of 4314 records were identified through database searching; excluding duplicates 3550 records were screened. A total of 3518 records excluded and 34 full-text articles were assessed for eligibility. Twelve full-text articles were excluded as they did not meet the eligibility criteria. Remaining 22 articles were included for further analysis [Figure 1].
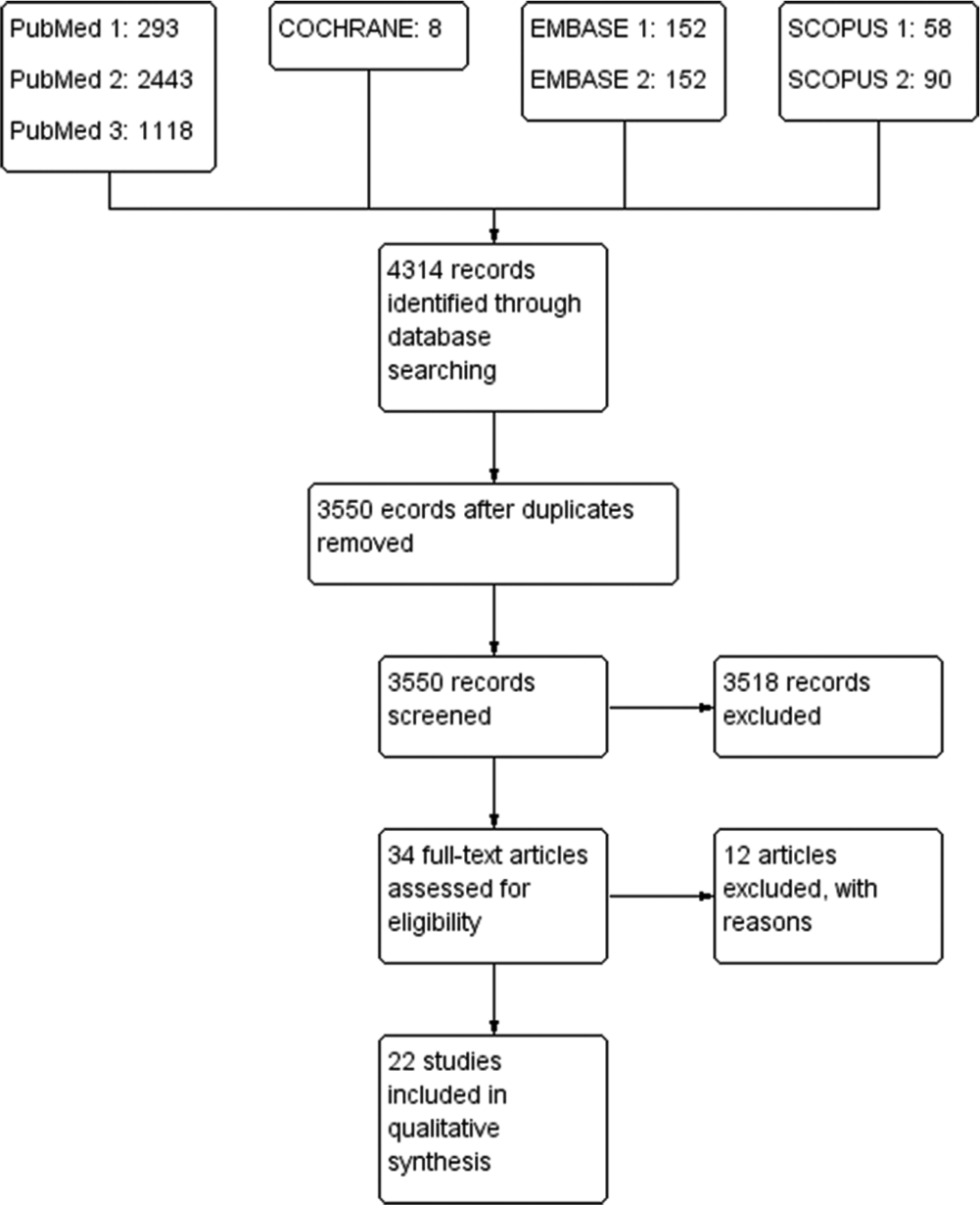
- Flowchart of selection of studies.
Study characteristics
A total of 22 studies met the eligibility criteria and they were included in the review. The data regarding the study title, year of study, authors name, year, country of origin, total number of patients, total number of patients with mGBM, mean age, gender, KPS/performance status (in any other reported scale), treatment details (extent of surgery/ radiotherapy/chemotherapy), and information on survival outcome were collected [Table 2]. A total of 8835 patients with mGBM were reported (17% of total cases), consisting of 57% of male patients. Biopsy was done in 34% of patients and 25% of patients underwent gross total excision. Mean survival was 9.1 months ± 2.5. Considerable variation was seen in reporting performance status and adjuvant therapy among the studies.
| Author name | Duration of study | Type of study | Total no of patients | mGBM patients | Mean age | Male | Female | KPS>70 | KPS≤70 | STB | Subtotal resection | GTR | RT | RT+CT | No. of patients received chemo | Overall survival mGBM | Statistical parameter | Overall survival sGBM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmadipour et al. | 2006–2014 | Retrospective | 565 | 231 | 62.2 | 11.4 | Mean | 13.5 | ||||||||||
| Armocida et al. | 2014–2016 | Retrospective | 176 | 12 | 64.2 | 5 | 7 | 9 | 3 | 3 | 3 | 12 | 10 | Median | 16 | |||
| Fleischmann et al. | 2011–2019 | Retrospective | 20 | 20 | 60.35 | 13 | 7 | 14 | 5 | 18 | 2 | 18 | 8 | Median | ||||
| Guerrini et al. | 2015–2018 | Retrospective | 16 | 16 | 66.5 | 11 | 5 | 12 | 3 | 5 | 10 | 2 | 9 | 8.7 | ||||
| Haque et al. | 2004–2016 | NCDB | 45268 | 7785 | <50=1022 51–65=3360, 65–79=2814, >80=589 | 4405 | 3380 | 2647 | 2731 | 2005 | 5958 | 4190 | 718 | 8.3 | Median | 12.8 | ||
| Kasper et al. | 2014–2019 | Retrospective | 183 | 54 | 68.5 | 38 | 16 | 8 | 27 | 5 | Median | 15 | ||||||
| Kong et al. | 2004–2010 | Retrospective | 51 | 20 | 53 | 31 | 20 | 10 | 10 | 14 | 16.03 | Median | 20.57 | |||||
| Lasocki et al. | 2007–2010 | Retrospective | 151 | 51 | 65 | 5.87 | Median | 11.53 | ||||||||||
| Liu et al. | 1997–2011 | Retrospective | 203 | 30 | 58.5 | 21 | 9 | 27 | 24 | 13 | 8 | Median | 11 | |||||
| Lou et al. | 2007–2008 | Phase II | 41 | 12 | 59.1 | 26 | 15 | 32 | 9 | 41 | 41 | 11.7 | Median | |||||
| Patil et al. | 2003–2010 | Retrospective | 368 | 47 | 62.7 | 36 | 11 | 27 | 16 | 4 | 41 | 36 | 6 | Median | 11 | |||
| Paulsson et al. | 2000–2010 | Retrospective | 161 | 33 | 63 | 20 | 13 | 12 | 14 | 7 | 33 | 28 | 8.2 | Median | 11 | |||
| Pérez-Beteta et al. | 2006–2017 | Retrospective | 97 | 97 | 62 | 50 | 47 | 42 | 55 | 6 | 63 | 5 | 7.39 | Median | ||||
| Syed et al. | 2004–2013 | Retrospective | 265 | 63 | 72.5 | 30 | 24 | 9 | 63 | 32 | 40 | 11.5 | Median | 14.8 | ||||
| Tunthanathip et al. | 2003–2018 | Retrospective | 173 | 30 | 54.5 | 19 | 11 | 14 | 16 | 9 | 19 | 2 | 22 | 8 | 6 | Median | 12 | |
| Wang et al. | 2015–2019 | Retrospective | 492 | 57 | 55.3 | 45 | 12 | 21 | 36 | 11 | 15 | 31 | 7 | 9 | 26 | 9 | Median | |
| Thomas et al. | 2006–2011 | Retrospective | 189 | 67 | 61 | 34 | 33 | 35 | 17 | 15 | 10 | Median | 18 | |||||
| Showalter et al. | 1995–2005 | Retrospective | 50 | 50 | 61 | 29 | 20 | 11 | 33% | 6 | 50 | 21 | 8.1 | Median | ||||
| Hassaneen et al. | 1993–2008 | Retrospective | 40 | 20 | 52 | 14 | 6 | 20 | 9 | 9 | 9.7 | Median | 10.5 | |||||
| Baro et al. | 2011–2021 | Retrospective | 98 | 98 | 62 | 63 | 35 | 26 | 45 | 0 | 2 | 54 | 26 | 10.2 | Median | |||
| Lahmi et al., | 2008–2017 | Retrospective | 11 | 11 | 50 | 7 | 4 | 11 | 9 | 2 | 11 | 10 | Median | |||||
| Dono et al., | 2004–2019 | Retrospective | 564 | 31 | 61 | 17 | 14 | 26 | 4 | 23 | 4 | 29 | 13 | Median | 17.9 |
m-GBM: Multiple GBM (multicentric or multifocal), sGBM=Solitary GBM, STB=Stereotactic biopsy, GTR: Gross total resection, NCDB: National cancer database
Description of individual studies
In the absence of randomized studies, most of the studies were retrospective in nature. A brief description of the included studies is presented below:
In a study by Ahmadipour et al.,[11] cohort of 565 patients of GBM (324 males/241 females) was reported (mean age: 62.2 years). Solitary lesion was present in 334 patients, multifocal lesion on one hemisphere in 183, and infiltration to contralateral lobe in 48 patients. Overall survival OS was 12.5 months. Overall survival in patients with infiltration of single lobe was 13.5 months compared to 11.4 months with multifocal single hemisphere infiltration, and 9.3 months with contralateral side infiltration. The authors reported that tumor infiltration of contralateral side had worse prognosis (adjusted odds ratio 2.1, P = 0.04).
In a retrospective single-center study, Armocida et al.[12] reported that completeness of resection was not significantly different between solitary or multifocal GBM. The study included 176 patients including 12 multifocal GBM. Overall survival of multifocal GBM was 10 months compared to 16 months for solitary GBM suggesting worse prognosis for mGBM.
Fleischmann et al.[13] defined multifocality as at least two independent contrast-enhancing foci in the MRI T1 contrast-enhanced sequence. In two cases, resection was performed, and in 18 cases, stereotactic biopsy was performed before the radiation therapy was started. Various dose fractionation regimens were used in the study. Total dose ranged from 50 to 60 Gy. Concurrent temozolomide (TMZ) was given in 18 cases. Median survival was 8 months (95% CI 3.6– 12.4 months) and median progression-free survival (PFS) after initiation of RT was 5 months (95% CI 2.8–7.2 months). The authors concluded that radiotherapy with concurrent TMZ is a potentially feasible treatment option for multifocal GBM.[13]
Guerrini et al.[6] reported OS of 8.7 months in mGBM (n = 16). Age ≤70 years, a post-operative KPS ≥70, gross or subtotal excision, and adjuvant treatment were shown to be associated with a significantly better prognosis.[6]
One of the largest studies was reported by Haque et al.[14] The authors evaluated demographic and clinical characteristics of solitary and mGBM from National Cancer Database (NCDB) analysis (2004–2016). Out of 45,268 total patients, 7785 (17.2%) had multifocal GBM. Gross total resection (GTR) (41.2% vs. 25.8%, P < 0.001), conventionally fractionated RT (48.2% vs. 42.7%, P < 0.001), and rate of surgery with biopsy only (24.1% vs. 34.0% P < 0.001) were different between the groups. Median OS was 12.8 months versus 8.3 months (P < 0.001) in cases with sGBM or mGBM, respectively. On multivariate analysis, unifocal disease, O(6)-methylguanine-DNA-methyltransferase (MGMT) methylation, radiotherapy, and chemotherapy were associated with improved overall survival.[14]
Kasper et al. reported significantly shorter OS for patients suffering from mGBM (1-year survival 64.9 ± 4.8% [sGBM] vs. 16.9 ± 6.4% [mGBM], P < 0.0001). The authors reported that on univariate analysis, completeness of resection, degree of tumor necrosis, adjuvant therapy, and proportion of tumor necrosis to initial volume were associated with improved overall survival. In multivariate Cox regression, however, only resection and adjuvant therapy retained statistical significance.[15]
In a study by Kong et al., total 20 out of 51 treatment naïve GBM patients had mGBM. Kaplan–Meier survival analysis suggested that multicentric mGBM patients had worse prognosis in comparison to solitary GBM (median, 16.03 vs. 20.57 months, P < 0.05). T1 contrast-enhanced and fluid-attenuated inversion recovery (FLAIR) images were used to define multicentricity.[16]
Lasocki et al.[4] studied improved characterization by FLAIR imaging and the prognostic significance of multifocality. In their study, the authors observed distinct contrast-enhancing lesions in 51 out of 151 GBM patients with interconnected lesions in 47 cases. Median overall survival of 176 days in mGBM compared to 346 days in solitary GBM (P = NS).
In a study by Liu et al.,[17] the clinicopathological and molecular features of 30 patients with mGBM was compared to 173 patients with solitary GBM. A total of 27 patients with mGBM underwent resection and 22 patients received radiotherapy. Median survival was 8 months compared to 11 months in solitary GBM.
Lou et al.[18] reported a Phase II study of upfront bevacizumab and Temoolomide (TMZ) in unresectable or multifocal GBM. All 41 patients underwent STB, and the cohort included 12 patients with mGBM. Unresectable tumors were included with the assumption that they have similar prognosis as multicentric disease. Following four cycles of therapy, surviving patients without progressive disease continued radiotherapy and chemotherapy with TMZ. Median overall survival of the entire cohort was 11.7 months (7.4–15.6 months).
Patil et al.[19] reported a case–control study of 47 patients in matched pair analysis design. Age, KPS score, and resection were found to be factors significantly affecting outcome in univariate analysis. In this study, multifocal tumors patients had significantly shorter (P = 0.02) median overall survival of 6 months versus 11 months. Two-year survival rates were 4.3% versus 29.0% (hazard ratio 1.8, 95% CI 1.1–3.1; P = 0.02).
Paulsson et al. reported the clinical outcome of a cohort of 41 patients with mGBM (33 multifocal and eight multicentric). Authors did not find any statistically significant difference in median overall survival between single versus multiple lesion GBM (11 vs. 8.2 months, P = 0.3) though median time to progression was more with sGBM (7.1 vs. 5.6 months, P = 0.02). No statistically significant difference in OS or PFS was noted between multicentric and multifocal GBM. No significant predictors among multiple lesion GBM (age, performance status, gender, multicentricity, and degree of resection) were noted on multivariate analysis.[20]
Pérez-Beteta et al. reported a radiological analysis of mGBM. In the cohort, the median survival was reported to be 7.39 months. Age, extent of surgery, contrast-enhancing rim width, and surface regularity were significant independent predictors of survival.[21]
Syed et al. reported the survival and recurrence pattern of multifocal GBM (63 out of 265 patients) after RT and mGBM had significantly worse survival (median OS = 11.5 vs. 14.8 months, P = 0.032). The authors found that multifocality was a poor predictor for PFS. Temozolomide therapy had a favorable effect on outcome.[8]
Concurrent TMZ therapy was found as strong predictor of outcome in another study by Tunthanathip et al.[22] The study reported clinical outcome of 30 mGBM (out of 173 GBM cased) patients. The median survival of the mGBMs was worse than sGBM (6 vs. 12 months, P = 0.003).
The beneficial effect of temozolomide chemotherapy on outcome of mGBM was also reported by Wang et al.[23] In this study, the authors showed CD8 + tumor-infiltrating lymphocytes was significantly lower in mGBM. In a cohort of 57 patients, the authors reported GTR in 31 patients. The authors reported a median OS of 9 months.
In the cohort (n = 189) reported by Thomas et al.,[3] median overall survival was 16.0 ± 1.3 months (sGBM = 18.0 ± 2.1 vs. mGBM = 10.0 ± 1.5, log rank P = 0.008). There was difference in outcome of multifocal and multicentric GBM (P = 0.009).
Showalter et al., reported a study of 50 patients of mGBM treated with radiotherapy either whole-brain RT or 3D conformal radiotherapy. The outcome was not different with two types of radiation. Median overall survival of the cohort was 8.1 months and time to progression 3.1 months.[7]
A study by Hassaneen et al.[24] reported outcome of 20 patients with multiple GBM in matched pair analysis. Mean age of presentation was 52 years and medial survival 9.7 months versus 10.5 months in sGBM (P = 0.34).
Baro et al.[25] reported outcome data of 98 patients of mGBM. Most of the patients were treated as per the standard EORTCNCIC trial protocol and median survival was 10.2 months. Concurrent chemoradiation with TMZ was shown to be a significant predictor of overall survival in this study.
Dono et al. reported overall survival of m-GBM shorter than s-GBM (13 months vs. 17.9 months, P = NS). The authors reported that 94% of the cohort was treated with EORTCNCIC protocol.[26]
Lahmi et al. reported median overall survival of 10 months (n = 11). Patients were treated with TMZ-based chemotherapy and whole-brain radiotherapy. Most of the patients underwent STB in the cohort.[27]
Radiological features of mGBM
Although glioblastoma mostly presents as solitary lesions on enhanced T1-weighted MRI, multiple enhancing lesions are increasingly recognized. Based on the appearance on FLAIR sequence, the multiple lesions can be multicentric or multifocal.[28] As many studies reported no pathologic or prognostic difference between multifocal and multicentric GBMs, we included both conditions as multiple GBM (mGBM) in this study. [Table 3] summarizes the radiological findings of various studies. In presented literature apparently, the uniform definition of multifocal diseases is not very well defined and most of the studies included in this review used a definition of mGBM based on MRI.[29] Overall edema and/or T2/FLAIR signal abnormality connecting between the lesions were reported in the studies,[4] and based on the defined criteria, multiple GBMs were categorized into either multicentric and multifocal GBMs.[17]
| Study ID | Details in MRI with contrast |
|---|---|
| Ahmadipour et al., 2019.[11] |
Tumor localization was determined based on contrast-enhanced T1-weighted sequences on axial and coronal images. Multifocality was divided (i) glioblastoma infiltration in a singular lobe, (ii) infiltration of>1 lobe within 1 hemisphere, and (iii) tumor infiltration of both hemispheres |
| Armocida et al., 2021[12] |
Tumors classified as Type I: Multicentric or multifocal supratentorial enhancing-contrast lesion at first diagnostic MRI Type II: Single enhancing contrast lesion |
| Baro et al., 2022[25] | Patients with multiple lesions were defined as those having at least two separate foci of enhancing tumor on MRI, separated by at least 1 cm |
| Fleischmann et al., 2021[13] |
Based on MRI with contrast-enhanced T1 and T2 or FLAIR sequences, only patients with multifocal growth pattern at the time of first diagnosis were included Multifocal: At least two independent contrast-enhancing foci in the MRI T1 contrast-enhanced sequence |
| Guerrini et al., 2021[6] |
To distinguish between MC and MF, FLAIR T2-weighted MRI sequences were used and in case a diffusion pathway was found between one or more lesions, the case was classified as a MF glioma. |
| Haque et al., 2020[14] |
Not mentioned |
| Hassaneen et al., 2011[24] |
Group A: Multifocal or multicentric glioblastomas, who underwent resection of all lesions through multiple craniotomies in a single session (patients with multifocal glioblastomas who were treated via a single craniotomy were excluded) two subgroups based on MR imaging-documented tumor characteristics Group A1 (multicentric lesions) widely separated lesions having no connection when visualized on FLAIR MR sequences and no identified route of dissemination Group A2 (multifocal lesions) multiple separate lesions seen to be connected on FLAIR sequences and/or there was evidence of leptomeningeal, subependymal, or CSF dissemination |
| Kasper et al., 2021[15] |
Multifocality was defined as separate (distance greater than 1 cm) contrast-enhancing lesions, independently from FLAIR hyperintensity. |
| Kong et al., 2016[16] |
This assessment was based on the patients’ MR contrast enhancement of T1-weighted images and FLAIR images. Multicentricity of the tumor was defined as the presence of multiple foci on the T1 contrast enhancement of MR images or having high signal for multiple lesions without contiguity of each other on the FLAIR image. |
| Lasocki et al., 2016[4] |
T2-weighted FLAIR and T1-weighted post-contrast sequences were used and interobserver agreement was assessed. Communication between lesions: The patients with more than 1 enhancing lesion were reviewed independently by the initial reader and a senior radiologist (with 8 years of subspecialty neuroradiology experience) Parenchymal spread: If there was evidence of continuous non-enhancing signal change between lesions involving the white and/or gray matter (including the corpus callosum), primarily based on the T2-weighted FLAIR sequence Subependymal and leptomeningeal spread: Based on the presence of separate enhancing lesions abutting the ventricular system or leptomeninges, respectively, without associated T2-weighted FLAIR signal abnormality in the intervening parenchyma If none of these three patterns of spread could be identified (i.e., no evident communication), the lesions were labeled multicentric |
| Liu et al., 2015[17] |
Pre-treatment MRIs of treatment naïve patients were used available in the National Cancer Institute’s The Cancer Imaging Archive (http://cancerimagingarchive.net/) S-GBM (Solitary glioblastoma) with one enhancing tumor M-GBM, with at least two clearly separated foci of enhancing tumors Multifocal and multicentric GBM. The centers of multicentric GBM belong to different lobes or bilateral brains, with no apparent route of dissemination. The centers of multifocal GBM may only be a short distance apart, suggesting that the tumor cells migrate elsewhere and develop into a new tumor center |
| Lou et al., 2013[18] |
|
| Patil et al., 2012[19] |
Patients with multifocal tumors were defined as those having at least two separate foci of enhancing tumor, separated by at least 1 cm. Twenty-seven (57.4%) of the 47 patients with multifocal disease had tumors located in the same cerebral hemisphere. Of the 20 patients who had tumors in both cerebral hemispheres, 13 were noted to cross the corpus callosum. Seven (14.9%) of the 47 multifocal tumors could be further classified as multicentric, with widely separated foci with no apparent route of dissemination. |
| Paulsson et al., 2014[20] |
Tumors were also classified as having multiple enhancing lesions, or whether any of the foci of tumor were non-enhancing tumors detected on T2 or FLAIR sequences |
| Pérez-Beteta et al., 2019[21] |
Multifocal glioblastomas: GBM with multiple foci, unconnected in post-contrast pre-treatment T1-weighted images |
| Showalter et al., 2007[7] |
Multifocal disease was defined as multiple tumor sites with clear separation between foci; Multicentric GBM: Lesions with>2 cm of separation or in contralateral lobes |
| Syed et al., 2018[8] |
mGBM was characterized as at least two non-connected foci of disease at least 1 cm apart from each other on magnetic resonance imaging Edema and/or T2/FLAIR signal abnormality was allowed to connect the gross tumor as per other studies |
| Thomas et al., 2013[3] |
Standard definition Sequences used: T1 pre-contrast sequence, T1 post-contrast sequence, and FLAIR sequence |
| Tunthanathip et al., 2020[22] |
Multiple GBMs were categorized into multicentric and multifocal GBMs. Multicentric GBMs were defined as those having at least two distinct foci of enhancing tumor with wide separation and without connecting T2/FLAIR signal abnormality Multifocal GBMs were clarified as the centers of the tumor connected |
| Wang et al., 2021[23] |
Standard definition followed |
| Lahmi et al., 2019[27] |
T1 with or without contrast enhancing, T2 flair |
| Dono et al., 2020[26] |
Standard definition was followed |
MRI: Magnetic resonance imaging, FLAIR: Fluid-attenuated inversion recovery
Prognostic factors
Various prognostic factors including age, pre-operative performance status (measured in different scales KPS, ECOG, Charlson-Deyo comorbidity score), extent of surgery, adjuvant treatment, chemoradiotherapy/radiotherapy, and use of TMZ have been evaluated in the literature. Various molecular markers including Ki67, MGMT methylation status, and IDH mutation have been evaluated but retrospective nature of studies with limited sample size and heterogeneity prevent from deriving any conclusive inference. Evidence will have to be based on prospective randomized trials conducted reducing heterogeneity and selection bias. Common prognostic factors that were analyzed in multivariate analysis in most studies were age, performance status, and extent of surgery, radiotherapy/ chemoradiotherapy, chemotherapy, MGMT, and isocitrate dehydrogenase 1 status. Cox proportional hazard model was most commonly used and the result of multivariate analysis from different studies is summarized in [Table 4]. Few studies included multifocality as one of the prognostic factors in multivariate analysis. Most of the studies in general showed poorer outcome of mGBM but small sample size is a limitation to quantify the risk. There was a trend for better survival in patients with at least one focus of non-enhancing FLAIR tumor but some studies did not find any statistically significant association of this prognostic factors.[20] A study by Thomas et al. did not identify single versus multiple lesion as independent predictor of outcome. Instead the authors reported difference in KPS and EOR as likely cause of difference in survival between m-GBM and s-GBM.[3]
| Authors | Criteria | HR | CI | P-value | |
|---|---|---|---|---|---|
| Age (years) | Syed et al. | >60 | 1.19 | 0.87–1.64 | 0.28 |
| Haque et al. | >80 | 2.602 | 2.45–2.76 | <0.001 | |
| 66–79 | 1.659 | 1.58-1.73 | <0.001 | ||
| 51–65 | 1.3 | 1.25–1.35 | <0.001 | ||
| (reference:≤50) | |||||
| Wang et al. | 0.636 | 0.306–1.321 | 0.225 | ||
| Dono et al. | >55 | 0.51 | −2.04 | 0.315 | |
| Performance status | |||||
| Syed et al. | KPS > 60 | 1.04 | 0.75–1.45 | 0.81 | |
| Haque et al. | Charlson-Deyo comorbidity score | ||||
| 1 | 1.2 | 1.17–1.24 | <0.001 | ||
| 2 | 1.34 | 1.19–1.29 | <0.001 | ||
| ≥3 | 1.46 | 1.39–1.55 | <0.001 | ||
| (ref 0) | |||||
| Showalter et al. | <70 versus ≥ 70 (KPS) | 2.42 | 1.14–5.14 | 0.022 | |
| Baro et al. | ECOG PS | ||||
| >2 versus 0–2 | 3 | 0.9–9.6 | 0.07 | ||
| Dono et al. | KPS > 80 | 1.32 | 0.36–4.80 | 0.677 | |
| Surgery | |||||
| Syed et al. | Any surgery | 1.28 | 0.95–1.73 | 0.1 | |
| Haque et al. | STR | 0.93 | 0.91–0.96 | <0.001 | |
| GTR | 0.74 | 0.72-0.76 | <0.001 | ||
| (reference: STB) | |||||
| Kasper et al. | EOR | 0.998 | 0.99–1.01 | 0.699 | |
| Showalter et al. | Biopsy versus GTR | 2.69 | 0.81–8.90 | 0.105 | |
| STR versus GTR | 1.6 | 0.58–4.41 | 0.364 | ||
| Salvage surgery (no vs. yes) | 5.47 | 1.48–20.21 | 0.011 | ||
| Radiotherapy | |||||
| Syed et al. | Chemoradiation | 0.89 | 0.67–1.19 | 0.44 | |
| Haque et al. | No radiation | 1.11 | 1.04–1.19 | 0.001 | |
| Conventional | 0.73 | 0.69–0.78 | <0.001 | ||
| Non-standard/not reported | 0.97 | 0.92–1.04 | 0.495 | ||
| Ref: Hypofraction | |||||
| Kasper et al. | Adjuvant therapy | 0.429 | 0.27–0.7 | 0.002 | |
| Tunthanathip et al. | TMZ + RT | ||||
| RT (ref) | 0.4 | 0.16–0.97 | 0.04 | ||
| Showalter et al. | RT type | ||||
| WBRT versus 3DCRT | 1.41 | 0.70–2083 | 0.331 | ||
| Baro et al. | Chemoradiation | ||||
| no versus yes | 3.1 | 1.3–7.7 | 0.014 | ||
| Dono et al. | Stupp protocol (temozolomide and radiation) | 0.09 | 0.006–1.39 | 0.086 | |
| Chemotherapy | |||||
| Haque et al. | No chemo | 1.29 | 1.24–1.33 | ≤0.001 | |
| Ref: Concurrent | |||||
| Wang et al. | Post op chemo | 6.076 | 2.33–15.84 | 0.0002 | |
| Showalter et al. | Salvage chemotherapy | 3.81 | 1.60–9.08 | 0.003 | |
| Dono et al. | Salvage bevacizumab | 0.55 | 0.14–2.10 | 0.382 | |
| Multifocality | |||||
| Syed et al. | 2 | 1.45–2.75 | <0.001 | ||
| Haque et al. | 1.396 | 1.36–1.43 | <0.001 | ||
| MGMT | |||||
| Haque et al. | Unmethylated | 1.41 | 1.34–1.49 | <0.001 | |
| Tunthanathip et al. | Methylated (ref: Unmethylated) |
0.4 | 0.05–3.23 | 0.39 | |
| Wang et al. | Methylated | 1.73 | 0.819–3.68 | 0.15 | |
| Baro et al. | Unmethylated versus methylated | 2.1 | 0.9–5.0 | 0.075 | |
| IDH mutation | |||||
| Tunthanathip et al. | Mutant IDH | 4.79 | 0.24–92.62 | 0.29 |
GTR: Gross total resection, STR: Sub-total resection, EOR: Extent of resection, KPS: Karnofsky performance status, MGMT: O (6)-methylguanine-DNA-methyltransferase, TMZ: Temozolomide, 3D-CRT: Three-dimensional conformal radiotherapy, WBRT: Whole-brain radiotherapy
DISCUSSION
In view of the advancement of MR imaging multiple lesions are more frequently reported than before.[4,30] It is generally accepted that mGBM has worse prognosis compared to GBM with singular lesion and has been shown in largest NCDB analysis.[14] The factors influencing the prognosis and outcome are poorly understood and there is no consensus in the existing literature regarding the optimal line of management. In a recently published systematic review, Li et al. summarized the challenges in diagnosis, management of mGBM and concluded that mGBM has poorer outcome which can be attributed to poor performance status at presentation, infiltrating nature of tumors making surgical resection challenging.[2] In this scoping review, we summarized the hazard ratio of prognostic factors most commonly included in multivariate analysis of retrospective studies and this will help in further design of randomized controlled trials. Clinically relevant retrospective studies, single-arm studies, case–control, and matched pair studies from 1993 were included in this scoping review in the absence of any randomized trials. Studies which were conducted with different primary objective (such as molecular markers and imaging end points) were hand searched and information regarding mGBM from these studies if they included cohort of mGBM patients, were collected. Therefore, there is heterogeneity among the studies which is a limitation of this review. We tried to include all such studies which provided overall survival information of mGBM. One of the points that need to be considered is that management of GBM changed after the publication of EORTC-NCIC trial which established concurrent chemoradiotherapy followed by adjuvant TMZ as standard of care for GBM.[31] As many patients with mGBM have poor KPS and a significant proportion undergo biopsy only, benefit of aggressive treatment regimen needs to be examined. As patients with single lesion are more likely to undergo gross total excision, it is likely that further adjuvant treatment may be decided by extent of resection (EOR).[3] In fact, maximum safe resection is one of the major factors that determine the prognosis and outcome.[24,32]
Multiple GBM is classified into two groups, multifocal and multicentric GBM. The foci of multicentric GBM are located in different lobes or bilateral brains, with no apparent connection in between. On the other hand, the centers of multifocal GBM may only be a short distance apart, are connected microscopically with the primary site or through commissural fibers, cerebrospinal fluid, or local extension.[33] [Figure 2] presents a proposed working classification. It has been hypothesized that multicentric GBM is biologically different from multifocal GBM and may evolve from two distinct locations in the brain.[3] The prognostic significance of biomarkers needs to be further explored. It has been suggested that the clinical presentation and prognosis of multicentric and multifocal GBM are not very different, and in this review, we have considered them together as multiple GBM.[25]
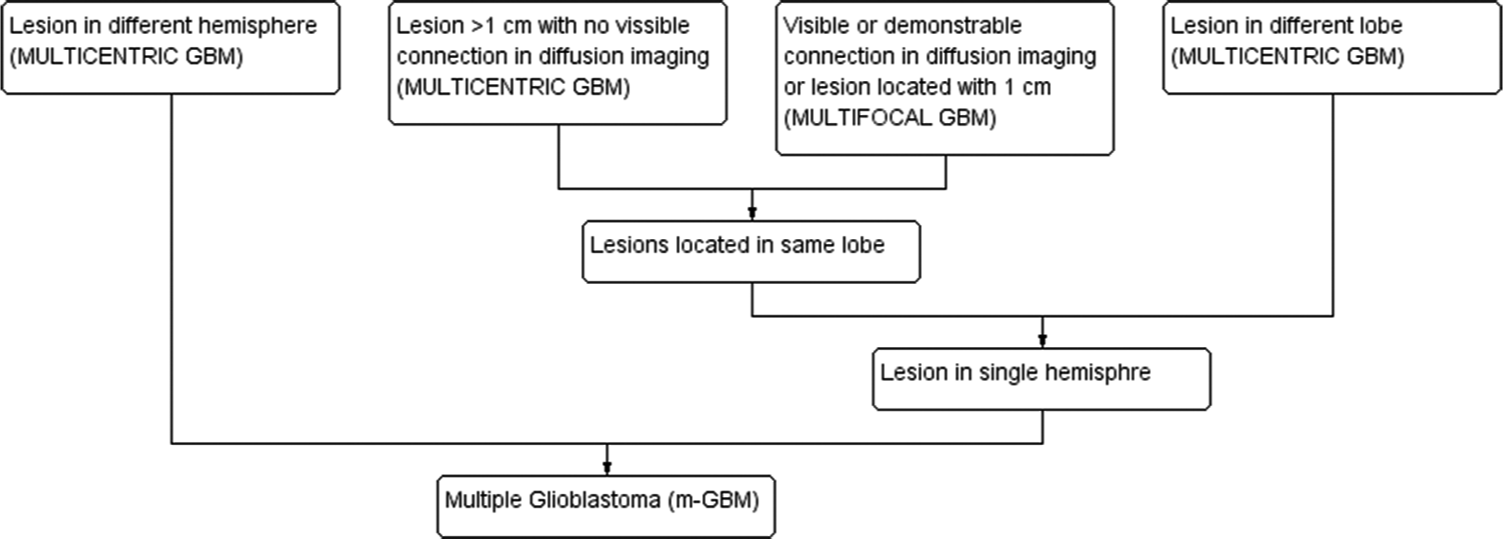
- Schematic classification of m-GBM.
All the studies included in this review were retrospective studies except for one Phase II study.[18] In the absence of RCT, it was only possible to systematically review retrospective studies. Recent studies have shown that radiotherapy and TMZ is an independent prognostic factor for survival, though the influence of extent of complete surgical resection is not clear.[25] Many studies have treated patients with radiotherapy alone though the exact reason is not very clearly stated. In addition to EOR, radiotherapy, and chemotherapy, various other factors including KPS, age, and biomarkers (MGMT, IDH, and Ki67) have been included in multivariate analysis. Except for some studies which have shown KPS to be a significant predictor, for most of the other factors, strength of association was not similar.
CONCLUSION
In view of the advancement of imaging, there is a much higher incidence of multiple GBM lesions at time of diagnosis than previously reported. Our study shows that mGBM has worse prognosis compared to GBM with singular lesion. As the factors influencing the prognosis and outcome is poorly understood and there is no consensus in the existing literature, this review is clinically relevant. As patients with single lesion are more likely to undergo gross total excision, it is likely that further adjuvant treatment may be decided by EOR. This review will be helpful for design of further prospective randomized studies to optimize the management of multicentric/multifocal GBM.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Multiple, primary brain tumors with diverse origins and different localizations: Case series and review of the literature. J Neurosci Rural Pract. 2018;9:593-607.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of multifocal and multicentric glioblastoma. J Clin Neurosci. 2021;83:71-6.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence and significance of multiple lesions in glioblastoma. J Neuro Oncol. 2013;112:91-7.
- [CrossRef] [PubMed] [Google Scholar]
- Multifocal and multicentric glioblastoma: Improved characterisation with FLAIR imaging and prognostic implications. J Clin Neurosci. 2016;31:92-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of multifocal gliomas. Oncology. 2010;79:306-12.
- [CrossRef] [PubMed] [Google Scholar]
- Is it worth considering multicentric high-grade glioma a surgical disease? Analysis of our clinical experience and literature review. Tomography. 2021;7:523-32.
- [CrossRef] [PubMed] [Google Scholar]
- Multifocal glioblastoma multiforme: Prognostic factors and patterns of progression. Int J Radiat Oncol Biol Phys. 2007;69:820-4.
- [CrossRef] [PubMed] [Google Scholar]
- Survival and recurrence patterns of multifocal glioblastoma after radiation therapy. Cancer Manag Res. 2018;10:4229-35.
- [CrossRef] [PubMed] [Google Scholar]
- Bevacizumab for patients with recurrent multifocal glioblastomas. Int J Mol Sci. 2017;18:2469.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of multifocality and molecular markers on survival of glioblastoma. World Neurosurg. 2019;122:e461-6.
- [CrossRef] [PubMed] [Google Scholar]
- Histological, molecular, clinical and outcomes characteristics of multiple lesion glioblastoma. A retrospective monocentric study and review of literature. Neurocirugia (Astur: Engl Ed). 2021;32:114-23.
- [CrossRef] [PubMed] [Google Scholar]
- Multifocal high-grade glioma radiotherapy safety and efficacy. Radiat Oncol. 2021;16:165.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of management and outcomes of unifocal versus multifocal glioblastoma. J Clin Neurosci. 2020;74:155-9.
- [CrossRef] [PubMed] [Google Scholar]
- On the Prognosis of multifocal glioblastoma: An evaluation incorporating volumetric MRI. Curr Oncol. 2021;28:1437-46.
- [CrossRef] [PubMed] [Google Scholar]
- Integrative radiogenomic analysis for multicentric radiophenotype in glioblastoma. Oncotarget. 2016;7:11526-38.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic, epigenetic, and molecular landscapes of multifocal and multicentric glioblastoma. Acta Neuropathol. 2015;130:587-97.
- [CrossRef] [PubMed] [Google Scholar]
- Phase II trial of upfront bevacizumab and temozolomide for unresectable or multifocal glioblastoma. Cancer Med. 2013;2:185-95.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosis of patients with multifocal glioblastoma: a case-control study. J Neurosurg. 2012;117:705-11.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of clinical outcomes and genomic characteristics of single focus and multifocal glioblastoma. J Neuro Oncol. 2014;119:429-35.
- [CrossRef] [PubMed] [Google Scholar]
- Morphologic features on mr imaging classify multifocal glioblastomas in different prognostic groups. AJNR. 2019;40:634-40.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical characteristics and prognostic factors of multiple lesions in glioblastomas. Clin Neurol Neurosurg. 2020;195:105891.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased CD8+lymphocytic infiltration in multifocal and multicentric glioblastomas. Front Oncol. 2021;11:748277.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple craniotomies in the management of multifocal and multicentric glioblastoma. J Neurosurg. 2011;114:576-84.
- [CrossRef] [PubMed] [Google Scholar]
- Newly diagnosed multifocal GBM: A monocentric experience and literature review. Curr Oncol. 2022;29:3472-88.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular characteristics and clinical features of multifocal glioblastoma. J Neuro Oncol. 2020;148:389-97.
- [CrossRef] [PubMed] [Google Scholar]
- Whole brain radiotherapy with concurrent temozolomide in multifocal and/or multicentric newly diagnosed glioblastoma. J Clin Neurosci. 2019;68:39-44.
- [CrossRef] [PubMed] [Google Scholar]
- T2/FLAIR Abnormity could be the sign of glioblastoma dissemination. Front Neurol. 2022;13:819216.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple glioblastomas: Are they different from their solitary counterparts? Asian J Neurosurg. 2015;10:266-71.
- [CrossRef] [PubMed] [Google Scholar]
- Non-contrast-enhancing tumor: A new frontier in glioblastoma research. AJNR Am J Neuroradiol. 2019;40:758-65.
- [CrossRef] [PubMed] [Google Scholar]
- Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-96.
- [CrossRef] [PubMed] [Google Scholar]
- A single-center retrospective safety analysis of cyclin-dependent kinase 4/6 inhibitors concurrent with radiation therapy in metastatic breast cancer patients. Sci Rep. 2020;10:13589.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term temozolomide might be an optimal choice for patient with multifocal glioblastoma, especially with deep-seated structure involvement: A case report and literature review. World J Surg Oncol. 2015;13:142.
- [CrossRef] [PubMed] [Google Scholar]






