Translate this page into:
Predicting Outcome in Skull Base Osteomyelitis: An Assessment of Demographic, Clinical, and Pathological Attributes
Birinder Singh Paul, DM Department of Neurology, Dayanand Medical College & Hospital Ludhiana, Punjab 141001 India drbirinder06@yahoo.co.in
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objective Skull base osteomyelitis (SBO) is an enigmatic clinical diagnosis which is difficult to decipher and is associated with poor outcomes. The study aims to examine the demographic and clinical characteristics of patients with SBO and its outcomes.
Materials and Methods Medical records of 30 patients with diagnosis of SBO over past 5 years were assessed for demographic and clinical characteristics, type of SBO, radiological parameters, treatment received, procedure performed, microbiological profile, comorbidities, and complications including cranial nerve (CN) palsies. These factors were analyzed for prediction of outcome (death or survival).
Statistical Analysis Microsoft Office Excel 2010 SAS 10.0 for Windows was used. Student's t-test for continuous variables (age, duration of symptoms, number of days of hospitalization, and treatment duration) and chi-square test for categorical variables (imaging findings, symptomatology, presence of comorbidities, surgical procedure, complications, and type of antibiotics) were utilized.
Results We found SBO was the disease of elderly population (64.07 ± 6.13 years) with male predominance (83.3%) highly associated with uncontrolled diabetes status (93.3%). Headache (100%) and CN palsy (80%) were the most common neurological presenting complaints followed by stroke (17%) and encephalopathy (10%). Pathological and radiological correlation showed that fungal infection (Aspergillus) was associated with anterior SBO (10%), while bacteria (Pseudomonas) was cultured from posterior SBO (30%). Fifty per cent of patients were alive after 1 year out of which 33% had good functional outcome. The mortality rate was 33.3% in our cohort and multiple lower CN palsies (p = 0.04), suboptimal duration of medical treatment (p = 0.03), surgical intervention during clinical course (p = 0.02), and development of intracranial or extracranial complications (p = 0.03) were the predictors of mortality.
Conclusion Early diagnosis including identification of pathogenic organisms and optimal duration of treatment are crucial factors for improved outcomes in SBO.
Keywords
skull base osteomyelitis
risk factors
outcomes
mortality
Introduction
Skull base osteomyelitis (SBO) is inflammation of the base of calvarium caused by microbial organisms.1 The skull base is composed of frontal, ethmoid, sphenoid, parietal, temporal, and occipital bones from anterior to posterior. It is divided by sphenoidal ridge anteriorly and petrous ridge posteriorly into three cranial fossae—anterior, middle, and posterior.2 The diagnosis of SBO can be made on radiological findings but requires a high degree of clinical suspicion. SBO still remains a diagnostic and therapeutic challenge as many clinicians are unaware of this condition and its long-term prognosis. Despite advancement in diagnostic and treatment modalities, the reported mortality is as high as 53%.3 The first review of chronic SBO was published in 1961 in only three patients, and highlights the rarity of the condition and difficulty in localizing the lesion.4 A retrospective analysis of five patients has emphasized the importance of prompt diagnosis and optimal duration of antibiotics in SBO.5
We report clinical manifestations, diagnostic characteristics, and functional outcomes in a series of 30 patients of SBO from a single tertiary care referral hospital. This is the first study to assess the functional outcomes and long-term survival in patients of SBO using standard neurological scales.
Materials and Methods
Study Population
We conducted a retrospective cohort study of 30 consecutive subjects who were admitted in the departments of neurology, neurosurgery, otolaryngology, and internal medicine in Dayanand Medical College and Hospital, Ludhiana, Punjab over a 5-year period (2015–2020). The study was undertaken with the approval from the local Institutional Ethics Committee (2020-544).
Case Definitions
The diagnosis of SBO was considered in patients who had osteomyelitis of clivus, petrous bone, or any part of base of skull confirmed on contrast-enhanced magnetic resonance imaging (MRI) of the brain or computed tomography (CT) of the head in addition to history of headache and cranial nerve (CN) palsies. Typical SBO or malignant otitis externa included osteomyelitis secondary to temporal bone infection, whereas when SBO was caused without any such preceding infection, it was classified as atypical.6 Anterior, central, and posterior SBOs were categorized according to skull base anatomy—anterior clinoid process and lesser wing of sphenoid constitute anterior, greater wing of sphenoid and petrous part of temporal bone denote central, and clivus involvement is indicative of posterior SBO.7 CN palsy was classified as single or multiple and in case of multiple, the patients were further categorized as predominantly upper CN palsy (I–VI) or lower CN palsy (VII–XII) or both. All patients with postoperative involvement of temporal bone flap and posttraumatic or iatrogenic osteomyelitis of skull base were excluded.
Parameters Assessed
The following information was extracted through patients' chart review and entered into a structured pro forma: clinical and demographic characteristics, type of SBO based on skull bones involved, and radiological data. Detailed neurological examination including CN palsies and other neurological deficits was also noted from patients' records. Any history of ear discharge or nasal symptoms, laboratory data including complete hemogram, blood cultures, erythrocyte sedimentation rate, and contrast-enhanced MRI of the brain and CT of the head were recorded. Hospital course including duration of stay, treatment received (antibiotics/antifungals, dosage, and duration), any diagnostic or therapeutic surgical interventions, and presence of any complication related to the disease developed during their hospital course were entered into the pro forma.
Outcomes Assessed
Outcome of all patients with the diagnosis of SBO was dichotomized as poor outcome (death) or survival. This was done by telephonic interview in 25 out of 30 patients as 5 could not be contacted and were lost to follow-up.
Standardized consent for analyzing and publishing the data was taken during the telephonic interview. In the patients with SBO who had died, we used the verbal autopsy questionnaire (shortened PHMRC [Population Health Metrics Research Consortium] verbal autopsy instrument) to ascertain whether the cause of death was related to SBO or to their previous comorbid conditions.8 Time duration between discharge and death was noted. For the survivors, functional outcome was assessed at 6 and 12 months with modified Rankin scale (mRS) and Glasgow outcome scale (GOS).9 10
Statistical Analysis
Statistical analysis was done with the help of Microsoft Office Excel 2010 SAS 10.0 for Windows. The age (years) of subjects, duration of symptoms, hospital stay, and treatment were noted as continuous variables. The presence of any comorbid condition, symptomatology, imaging findings, any procedure done, complications during hospital course, and type of antibiotics were considered as categorical variables.
Continuous variables were demonstrated as mean ± standard deviation. Student's t-test for continuous variables and chi-square test for categorical variables were used to chart the comparisons of these variables between those who died or survived.
Results
Demography
In this cohort of 30 patients, 25 (83.3%) patients were males with mean age of 64.07 ± 6.13 years. Diabetes mellitus was the most common comorbid condition (93.3%) with others being hypertension, chronic kidney disease, chronic liver disease, and coronary artery disease. Baseline and demographic characteristics of the cohort are described in Table 1.
|
Baseline characteristics |
Number of patients (%) N = 30 |
|---|---|
|
Gender |
|
|
Male |
25 (83.3) |
|
Female |
05 (16.6) |
|
Mean age (y) |
64.07 ± 6.13 (46–81) |
|
Side of SBO |
|
|
Right |
14 (46.6) |
|
Left |
14 (46.6) |
|
Bilateral |
02 (6.6) |
|
Clinical complaints |
|
|
Headache |
30 (100) |
|
Neurological examination |
|
|
Cranial nerve palsies |
|
|
Upper (I–VI) |
02 (6.6) |
|
Lower (VII–XII) |
24 (80) |
|
Both set of cranial nerves |
04 (13.3) |
|
Comorbidities |
|
|
Diabetes mellitus |
28 (93.3) |
|
Hypertension |
18 (60) |
|
CKD |
06 (20) |
|
CLD |
03 (10) |
|
CAD |
03 (10) |
|
Systemic vasculitis |
02 (6.6) |
|
Intracranial complications |
|
|
Stroke |
11 (36.6) |
|
Anterior circulation infarct |
04 (13.3) |
|
Brain stem infarct |
01 (3.3) |
|
Bezold's abscess |
01 (3.3) |
|
Encephalopathy |
03 (10) |
|
Temporomandibular joint involvement |
02 (6.6) |
|
ESR |
78.26 ± 27.71 (09–132) |
|
Procedure |
|
|
Tympanomastoid procedure |
12 (40) |
|
Mastoidectomy |
09 (30) |
|
Diagnostic biopsy (FESS) |
03 (10) |
|
Radiological profile |
|
|
Atypical |
24 (80) |
|
Typical |
06 (20) |
|
Anterior |
03 (10) |
|
Posterior |
09 (30) |
Abbreviations: CAD, coronary artery disease; CKD, chronic kidney disease; CLD, chronic liver disease; ESR, erythrocyte sedimentation rate; FESS, functional endoscopic sinus surgery; SBO, skull base osteomyelitis.
Clinical Characteristics
Headache was the presenting symptom in 30 patients (100%). All patients with SBO had CN palsies either single or multiple. Ten (33.3%) patients had one CN involvement, while 20 (66.6%) had more than one CN involvements. Lower CNs (VII–XII) were more commonly involved (80%).
Imaging Findings
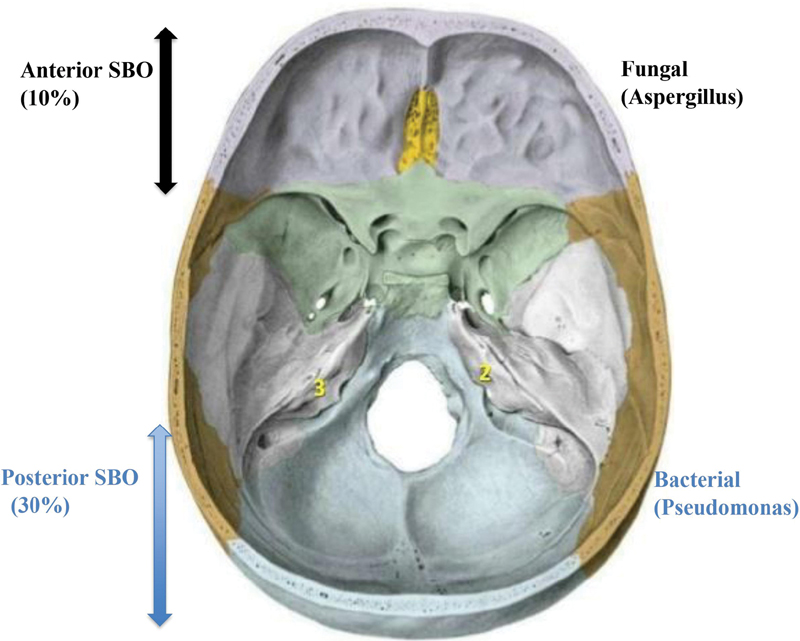
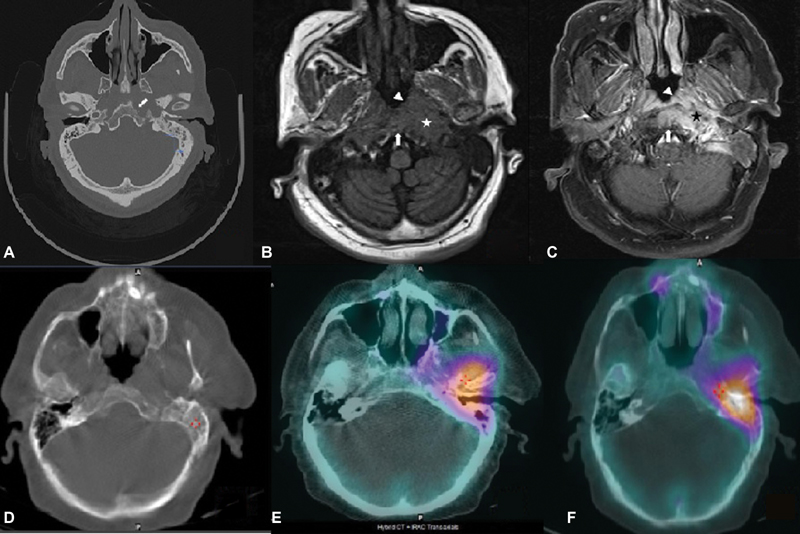
According to MRI, 18 (60%) patients had central (atypical), 9 (30%) had radiological findings suggestive of posterior, while 3 (10%) patients had anterior SBO (Fig. 1). Single photon emission computed tomography/CT was done on one patient and it revealed three foci of osseous overactivity in mastoid process of left temporal bone with extension into middle ear, petrous apex, and left temporomandibular (TM) joint (Fig. 2).
-
Fig. 1 Classification of skull base osteomyelitis (SBO) along with causal microorganisms.
Fig. 1 Classification of skull base osteomyelitis (SBO) along with causal microorganisms.
-
Fig. 2 First row image: (A) Axial computed tomography (CT) demonstrates erosion of cortex along left lateral clivus and left petrous apex (arrow), (B) axial T1-weighted magnetic resonance imaging, and (C) magnetic resonance postcontrast T1 show infiltrative soft tissue in submucosa of nasopharynx on left side (arrowhead) extending posteriorly to paraclival tissue and left carotid space (star); in addition, marrow of clivus and basiocciput was involved by infective process (arrow). Second row image: (D) Bone CT shows destruction of left mastoid, (E) bone single photon emission computed tomography showing high-grade uptake in left petrous apex, and (F) left temporomandibular joint.
Fig. 2 First row image: (A) Axial computed tomography (CT) demonstrates erosion of cortex along left lateral clivus and left petrous apex (arrow), (B) axial T1-weighted magnetic resonance imaging, and (C) magnetic resonance postcontrast T1 show infiltrative soft tissue in submucosa of nasopharynx on left side (arrowhead) extending posteriorly to paraclival tissue and left carotid space (star); in addition, marrow of clivus and basiocciput was involved by infective process (arrow). Second row image: (D) Bone CT shows destruction of left mastoid, (E) bone single photon emission computed tomography showing high-grade uptake in left petrous apex, and (F) left temporomandibular joint.
Hospital Course
Procedure
Only 12 patients underwent surgical intervention. Cultures revealed growth of Pseudomonas in nine (30%) patients, while two out of three (10%) patients who had radiological findings of anterior SBO had fungal growth in their diagnostic endoscopy biopsy.
Complications
In our study, 11 (36.6%) patients suffered additional intracranial complications, 4 had anterior circulation stroke, while 1 had posterior circulation stroke. Encephalopathy was observed in one patient. One patient developed Bezold's abscess, while right TM joint osteomyelitis was noted in two patients.
Treatment Regimens
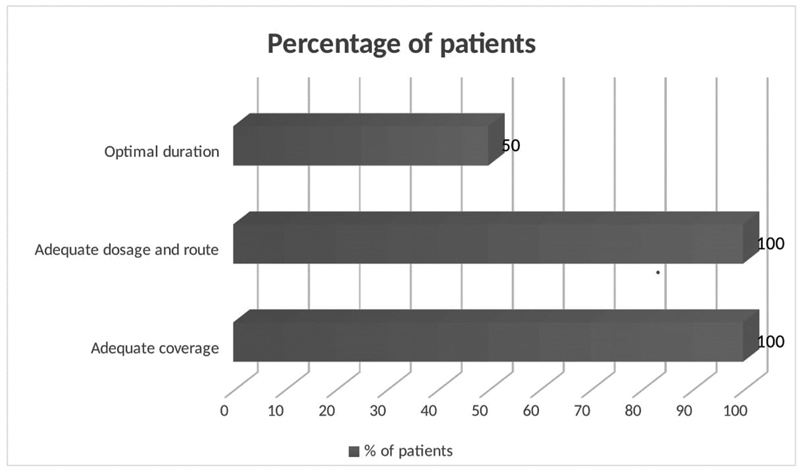
All patients were treated with intravenous broad-spectrum antimicrobials to cover entire range of putative microorganisms during their hospital stay which included piperacillin–tazobactam in 18 (60%), carbapenems in 6 (20%), cephalosporins in 5 (16.6%), and vancomycin in 1 (3.3%) patient. They were advised to continue oral antimicrobials following discharge; however, only half of patients took treatment for sufficient time period (6–20 weeks).11 The mean duration of treatment was 71 days (range: 4–210 days). Patients were categorized as having received adequate treatment if they fulfilled the parameters of treatment as given in Fig. 3.
-
Fig. 3 Percentage of patients who received adequate treatment.
Fig. 3 Percentage of patients who received adequate treatment.
Outcomes
Outcome assessment was possible in 25 patients as 5 were lost to follow-up. The mortality rate in our cohort was 33.3% out of which two patients died in hospital in whom the cause of death was directly related to SBO, that is, aspiration pneumonitis due to bulbar palsy, while eight patients died after discharge from hospital. After discharge, the cause of death as determined with the help of Verbal Autopsy Questionnaire was aspiration in four (55%) and neurologically deteriorating condition in three patients and one patient who was a recipient of renal transplant died due to sepsis. The mean duration of survival among those who died after discharge was 43 days. After 1 year, 15 (50%) patients of SBO were alive among whom 10 (33.3%) showed good functional outcome as per the neurological scales—mRS <3 and GOS >1, while 5 needed some form of assistance in their routine daily activities.
Discussion
SBO is a complex pathological condition associated with a high morbidity and mortality despite advancements in diagnostic modalities and management techniques. The diagnosis of disease is challenging because there are no set diagnostic criteria. In this study, we tried to access the knowledge gap in SBO by addressing its clinical, laboratory, and radiological characteristics and emphasize the factors affecting survival.
Poorly controlled diabetes mellitus with duration more than 5 years was the major comorbid condition associated with SBO in our cohort (93.3%). There was high frequency of SBO in elderly in their sixth decade of life (mean age 64 years) with a male preponderance (83.3%). Two patients, one with vasculitis and other on immunosuppressants were younger in the cohort (46 and 52 years). Headache was the most common symptom in all patients and the presenting symptom in 80%. Multiple CN palsies was the second most common neurological finding, lower CN palsy being more commonly seen (80%) than upper (6.6%). One patient had six CN palsies (VII–XII). The presence of multiple lower CN palsies was associated with increased mortality (p = 0.04). In our study, we found 24 (80%) patients had atypical SBO. The major neurological complications associated in these patients were stroke (17%) and encephalopathy (10%), while distant noncontiguous abscess (3%) and infective arthritis of TM joint (7%) were also observed. The presence of any of these complications was a strong predictor of mortality (p = 0.03) and this has not been reported previously. Diagnostic or therapeutic procedure was done in 12 (40%) and pathogen was cultured in 11 (37%) patients. Though infectious process in SBO can be caused by wide range of pathogens; however, in our study, Pseudomonas was isolated from nine (30%), while Aspergillus from two (7%) patients. On pathogenic and radiological correlation, Aspergillus was more commonly associated with anterior (10%) and Pseudomonas with posterior SBO (30%). Poor outcome was noted in patients in whom the aforementioned procedure was done (p = 0.02). In our cohort, 24 (80%) patients were discharged on home-based care. On critically appraising the treatment, 50% received suboptimal treatment (duration <6–20 weeks). On further analysis, we found that inadequate treatment was the key factor for poor outcome (p = 0.03). After 1 year follow-up, 50% patients with SBO were alive out of which 33% had good functional outcome (mRS <3 and GOS >1). Mortality rate noted in our cohort was 33%.
Poorly controlled glycemic status leads to poor microcirculation, causing endarteritis and impaired immunity, also advancing age with presence of comorbidity leads to decreased bone vascularity and hence, increasing the susceptibility for infections.12 13 Headache in SBO is related to dural inflammation, bone destruction, or abscess formation.14 The presence of lower CN palsy may be related to spread of infection beyond the temporal bone into posteromedial area of skull base resulting in involvement of jugular foramen and paralysis of IX to XI CNs. Cerebrovascular accident may be related to invasion of the blood vessels by infection leading to vasculitis.12 Also, enhanced atherosclerosis due to advanced age and presence of comorbidities such as diabetes mellitus or chronic kidney disease may also be the implicating factors. Extension of infection into the infratemporal fossa and deep cervical fascia may have led to the formation of Bezold's abscess. In the current study, poor outcome was noted even after the therapeutic or diagnostic procedure as compared with those who had no procedure (p = 0.02). Though debridement of necrotic bony tissue was done in only 40% of patients, remaining had diagnostic procedure, still this could not prevent the disease from progressing. However, this does not suggest that surgery by itself was not useful, in fact patients who needed the procedure were already in advanced stage of the disease. Also, debridement may itself expose the healthy bone to invading pathogens causing aggressive disease progression.15
The high frequency of SBO in elderly male diabetics was also reported previously.16 17 Previous studies have reported isolated VII or VIII CN palsy which may be because of inclusion of patients with malignant otitis externa.18 On the contrary, in our cohort, majority had atypical SBO (80%) with no obvious clinical foci of infection elsewhere. Venous thrombosis is said to be more commonly associated with SBO, but we did not encounter any such patient.19 Rather, we had patients with anterior and posterior circulation strokes and presence of any such complication was a strong predictor of mortality (p = 0.03). MRI and CT scans were the most important radiological modalities for diagnosis, classification of SBO, and establishing the extent of disease. Chandler et al reported four cases, while Singh et al published 10 patients with atypical SBO diagnosed on contrast-enhanced MRI showing lytic lesion involving clivus, sphenoid, and petrous bones in the absence of malignant otitis externa.3 20 Literature recommends aggressive treatment for minimum of 6 to 20 weeks due to poor vascularity of target area and impaired immune response associated with underlying comorbidities.11 12 Mortality rate noted in our cohort was 33% as compared with 10 to 20% in previous literature.21
Predictors of mortality in patients with SBO were presence of multiple lower CN palsies (p = 0.04), surgical intervention during their clinical course (p = 0.02), suboptimal duration of medical treatment (p = 0.03), or those who developed intracranial or extracranial complications (p = 0.03) as shown in Table 2. The presence of these factors in combination or isolation signifies extensive infectious process involving bones of skull base, foramina, soft tissue, as well as blood vessels. In addition, if duration of antibiotics was not adequate, it becomes difficult to curtail the infection which leads to persistent disease hence increased chances of mortality.
|
Outcome |
Chi-square value |
p-Value |
Odds ratio (95% CI) |
|||
|---|---|---|---|---|---|---|
|
Alive n (%) |
Death n (%) |
|||||
|
Age group (y) |
< 60 |
6 (40) |
3 (30) |
0.260 |
0.610 |
1.56 (0.28–8.53) |
|
> 60 |
9 (60) |
7 (70) |
||||
|
Gender |
Female |
2 (13) |
3 (30) |
1.042 |
0.307 |
0.36 (0.05–2.68) |
|
Male |
13 (87) |
7 (70) |
||||
|
Cranial nerve |
Lower |
10 (67) |
10 (100) |
4.167 |
0.041 |
|
|
Upper |
5 (33) |
|||||
|
Treatment adequacy |
No |
9 (60) |
10 (100) |
5.263 |
0.031 |
|
|
Yes |
6 (40) |
|||||
|
Complication |
No |
12 (80) |
3 (30) |
6.250 |
0.034 |
9.33 (1.47–59.48) |
|
Yes |
3 (20) |
7 (70) |
||||
|
Procedure |
No |
13 (87) |
4 (40) |
6.005 |
0.028 |
9.75 (1.38–68.78) |
|
Yes |
2 (13) |
6 (60) |
||||
Abbreviation: CI, confidence interval.
Note: p-values in bold are statistically significant (p < 0.05).
Our study is not without limitations. The retrospective data collection of 30 patients may have introduced a recording bias. This may be because the incidence of disease is low. The follow-up of patients was done telephonically and no advanced neurosurgical procedure was performed during the hospital stay. Since our aim was to assess mortality rather than radiological cure, follow-up MRI was not done.
Conclusion
In our study, mortality was related to lower CN palsies (VI–XII), presence of neurological complications, inadequate treatment, and any surgical intervention, whereas age, gender, presence of diabetes mellitus or any other comorbidity, and type of SBO (typical or atypical) did not predict mortality. The optimal treatment and its duration are still a perplex issue and evidence-based studies are needed to guide the clinicians in this regard. Our attempt to study functional outcomes in patients with the help of standardized neurological scales can prove to be a useful tool for clinicians to monitor patients on treatment of SBO. The most critical factor that holds utmost importance is the clinching of diagnosis at an early stage and sustained prolonged treatment with monitoring of compliance for patients of SBO.
Acknowledgment
The authors would like to thank their patients for participating and sharing their data for this study.
Conflict of Interest
None declared.
Funding None.
References
- Imaging of skull base: pictorial essay. Indian J Radiol Imaging. 2012;22(4):305-316.
- [Google Scholar]
- Central skull base osteomyelitis: diagnostic dilemmas and management issues. Indian J Otolaryngol Head Neck Surg. 2016;68(2):149-156.
- [Google Scholar]
- Central skull base osteomyelitis: new insights and implications for diagnosis and treatment. Eur Arch Otorhinolaryngol. 2015;272(5):1269-1276.
- [Google Scholar]
- A shortened verbal autopsy instrument for use in routine mortality surveillance systems. BMC Med. 2015;13:302.
- [Google Scholar]
- Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091-1096.
- [Google Scholar]
- The Glasgow outcome scale - 40 years of application and refinement. Nat Rev Neurol. 2016;12(8):477-485.
- [Google Scholar]
- A comprehensive review of skull base osteomyelitis: diagnostic and therapeutic challenges among various presentations. Asian J Neurosurg. 2018;13(4):959-970.
- [Google Scholar]
- Outcomes of malignant external otitis: survival vs mortality. Acta Otolaryngol. 2010;130(1):89-94.
- [Google Scholar]
- Skull base osteomyelitis: factors implicating clinical outcome. Acta Neurol Belg. 2019;119(3):431-437.
- [Google Scholar]
- Dural and pial pain-sensitive structures in humans: new inputs from awake craniotomies. Brain. 2018;141(4):1040-1048.
- [Google Scholar]
- Malignant external otitis: review and personal experience. Acta Otolaryngol Suppl. 1996;521:3-16.
- [Google Scholar]
- Central or atypical skull base osteomyelitis: diagnosis and treatment. Skull Base. 2009;19(4):247-254.
- [Google Scholar]
- Temporomandibular joint disorder from skull-base osteomyelitis: a case report. Maxillofac Plast Reconstr Surg. 2015;37(1):39.
- [Google Scholar]
- Malignant otitis externa: causes for various treatment responses. J Int Adv Otol. 2020;16(1):98-103.
- [Google Scholar]
- Clinical characteristics and complications of skull base osteomyelitis: a 12-year study in a teaching hospital in South India. J Family Med Prim Care. 2019;8(3):834-839.
- [Google Scholar]
- Clinical profiling and management outcome of atypical skull base osteomyelitis. Br J Neurosurg. 2020;34(6):686-689.
- [Google Scholar]
- Severe skull base osteomyelitis caused by Pseudomonas aeruginosa with successful outcome after prolonged outpatient therapy with continuous infusion of ceftazidime and oral ciprofloxacin: a case report. J Med Case Reports. 2017;11(1):48.
- [Google Scholar]






