Translate this page into:
Pediatric stiff-person syndrome with renal failure
Address for correspondence: Dr. M. Veerendra Kumar, Government Medical College, Kottayam, Kerala - 686 008, India. E-mail: vkumarbabu@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Stiff-person syndrome (SPS) is an autoimmune neuronitis with progressive myoclonus and stiffness. It is a rare but treatable disorder with few case reports in children. SPS is due to autoantibodies against the enzyme glutamic acid decarboxylase which is present in neuronal and nonneuronal tissues. This is the case report of an 8-year-old boy with clinical and investigational features suggestive of SPS with associated myoglobin-induced renal failure, who completely recovered with treatment.
Keywords
Glutamic acid decarboxylase
myoclonus
rituximab
stiff-person syndrome
Introduction
Stiff-person syndrome (SPS) is an autoantibody-mediated disease against the GABAergic (Gamma-Amino Butyric Acid) neurons.[1] Due to decreased GABA neuron function, which normally is inhibitory, there will be increased stiffness and spasm of muscles. Autoantibody against glutamic acid decarboxylase (GAD) in serum or cerebrospinal fluid (CSF) is supportive for the diagnosis.[12] Pediatric case reports of this condition are less in number.[3] Its true frequency has not been fully ascertained. The British Neurological Surveillance Unit identified 119 cases among the UK population over 5 years (2000–2005) implying a prevalence of 1–2 cases per million, of which 5% was seen in children.[2]
Case Report
An 8-year-old male child born to nonconsanguineous parents came to us, with a 2 months history of progressively increasing jerky movements of body and limbs. This began on right leg, as sudden flexor spasms of the foot while walking, which led to frequent falling. In 2 weeks’ time, it increased in frequency and spread to right thigh muscles as flexion contractions of the hip. By 1½ months, trunk muscles and upper limbs were also affected. Extensor spasms predominated in trunk muscles which resulted in frequent prolonged arching back of the body. Due to these myoclonic contractions he could not sit or handle things. Spasms were painful, present during rest, increased by activity, touch, loud sound, and disappeared in sleep. His sensorium was normal. He had no difficulty in chewing or swallowing food. No bowel or bladder retention or incontinence. No sensory involvement. He developed oliguria and high colored urine in the last 1-week. No history of any serotoninergic or antidopaminergic drug intake and no difficulty in opening mouth.
During examination, he had very frequent, violent myoclonic jerks of trunk and limbs. Due to severe lordosis he could not lie supine. He was in agonizing pain during the spasms and had visible severe wasting. Pulse rate 83/min, blood pressure 130/76 mmHg, respiratory rate 18/min, and had mild pedal edema. There were multiple pressure sores on areas where there was frequent rubbing of the skin. Higher mental functions and cranial nerves were within normal limits. Muscle tone was increased and passive movements were difficult.
Superficial reflexes were normal including flexor plantar. Deep tendon reflexes were brisk. All sensations were normally appreciated. No cerebellar signs. Skull and spine were normal. Fundus examination revealed no abnormality. We made a provisional diagnosis of subacute encephalomyelitis and myoglobin mediated acute tubular necrosis with differential diagnosis of SPS and progressive myoclonic epilepsy.
His blood counts, serum calcium, thyroid function, blood sugar, glucose tolerance test, and tuberculosis screening, were within normal limits. Magnetic resonance imaging brain, spine, and electroencephalography (EEG) also were normal. Investigation results, which were abnormal, are given in the Table 1. X-ray chest, ultrasound sonography abdomen did not reveal lymphadenopathy or other neoplastic lesions. All investigations were sent in the first 2 days of admission based on the initial differential diagnosis.
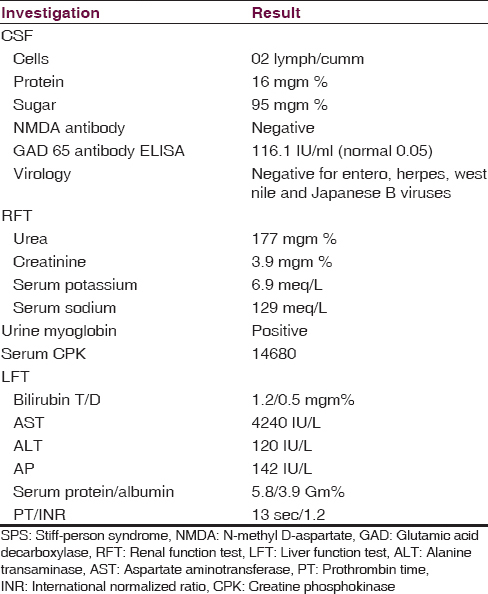
He was treated with alkalinization and potassium lowering agents. Renal function started improving from next day. Methylprednisolone intravenous (IV) was given for 5 days along with clonazepam and valproate. Since there was no improvement, IV immunoglobulin (IVIG) was given for 5 days. There was no change in myoclonus. By then, the result of CSF autoantibody GAD65 came as positive. Hence, he was started on IV rituximab 375 mgm/m2/week. After the second dose of rituximab, myoclonus decreased significantly, he started sitting comfortably and began walking with little support in the next 7 days.
His renal function completely returned to normal. A total of 4 doses of rituximab was given at weekly intervals. He was discharged on oral tapering dose of prednisolone, clonazepam, and sodium valproate. He was doing well at 1-month follow-up visit. His muscle spasms had completely subsided.
Discussion
Myoclonus is a movement disorder, which presents itself with sudden, brief, shock-like jerks. Normal EEG is a pointer against progressive myoclonic epilepsy and degenerative disease associated myoclonic seizures.[4] No detectable neoplastic lesions, is against paraneoplastic immunologic events, even though long-term follow-up is required to certainly rule out neoplasm. The absence of, altered sensorium, neuropsychiatric symptoms, and choreoathetoid movements were pointers against other autoimmune encephalitis.[5] No history of any drug intake ruled out drug-induced dystonia. Absence of lock jaw pointed against tetanus. Normal imaging studies of brain and spine with absent CSF pleocytosis, prompted us to send the CSF for the most common neuro autoantibodies in children – N-methyl D-aspartate (NMDA) and GAD65.[6]
We made the final diagnosis after investigations, as SPS with myoglobin-induced acute tubular necrosis. Barker et al. divided SPS into three categories: The classical SPS, the stiff limb syndrome (c/c spinal interneuronitis), and progressive encephalomyelitis with rigidity.[2] Our child had classical SPS, since he had predominant axial involvement and auditory stimulus sensitivity of muscle spasms.[4] The dalakas criteria are used worldwide to diagnose SPS.[2] Following five are the components of the criteria.[1] Stiffness in the axial muscles, prominently in the abdominal and thoracolumbar paraspinal muscles leading to a fixed deformity (hyperlordosis).[2] Superimposed painful spasms precipitated by unexpected noises, emotional stress, tactile stimuli.[3] Confirmation of the continuous motor unit activity in agonist and antagonist muscles by electromyography.[4] Absence of neurological impairments that could explain the stiffness.[5] Positive serology for GAD65 (or amphiphysin) autoantibodies, assessed by immunocytochemistry, western blot or radioimmunoassay. Reported child had 4 out of 5 parameters of the above criteria. We could not do electromyogram. Other autoantibodies and GAD65 epitope binding studies were not done due to financial constraints and lack of easy availability. GAD autoantibody titer in serum or CSF does not correlate with symptom severity, and so titer monitoring is unnecessary.[2]
Autoantibody GAD is also found in non-neuronal tissues, such as beta cells of pancreatic islets, thyroid, thymus, testes, and oviducts. Thirty percent of patients with SPS have insulin-dependent diabetes and both diseases share autoantibodies to the same GAD65 with the difference in epitope binding. Our child did not have nonneuronal involvement.
As it is an autoimmune disorder, steroids and IVIG, either alone or combined, is the first line immunotherapy.[2] Since he did not show improvement after 10 days, we used the second line immunosuppressant, IV rituximab.[2] After the second dose of rituximab, there was a significant decrease in spasms. Plasma exchange is another option but not well established in SPS.[2]
We are reporting this case, to include SPS in the differential diagnosis of any child with myoclonic jerks, normal sensorium, and absent pyramidal signs with normal EEG.
In all children with frequent muscle spasms, renal function also needs to be tested, since myoglobin released from overacting muscles can cause acute tubular necrosis.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Myoclonic disorders: A practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4:47-62.
- [Google Scholar]
- Paediatric autoimmune encephalopathies: Clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry. 2013;84:748-55.
- [Google Scholar]






