Translate this page into:
Pediatric multifocal glioblastoma multiforme with fulminant course
Address for correspondence: Dr. Goutham Cugati, Department of Neurosurgery, Dr. Achanta Lakshmipathi Neurosurgical Centre, Post Graduate Institute of Neurological Surgery, V.H.S Hospital, Taramani, Chennai, India. E-mail: gcugati@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor. GBM in children is less common than in adults and has a better prognosis. Pediatric GBM is a rare entity, and a multifocal development in a pediatric GBM is much rarer. We report to you one such rare case of pediatric multifocal GBM in a 5-year-old child who developed rapidly increasing multiple lesions after radiotherapy. More studies are required to study the genetic analysis, tumor behavior, management and outcome of these rare tumors.
Keywords
Multifocal GBM
multicentric GBM
pediatric GBM
recraniotomy
Introduction
The incidence of glioblastoma multiforme (GBM) is less common in children than in adults. The prognosis in children appears to be better than the adult counterpart as seen in various studies.[1–3] Multiple lesions in GBM are found in 2 instances: Multifocal and multicentric. A GBM is said to be multifocal when there are multiple lesions and there is an established route of spread or dissemination between these lesions. The following case report is about a 5-year-old child with a multifocal GBM.
Case Report
This 5-year-old girl presented with history of tonic clonic seizures for 8 months. Magnetic resonance imaging (MRI) of the brain revealed right temporo-parietal mixed intensity lesion, which was predominantly cystic. Patchy ill-defined enhancement was noted with midline shift and subfalcine herniation [Figure 1]. Magnetic resonance spectroscopy showed significant increase in choline and lactate peaks. She underwent right temporo-parietal craniotomy and gross total excision of the tumor. Histopathological examination (HPE) was suggestive of glioblastoma multiforme. Tumor cells were glial fibrillary acidic protein (GFAP) positive and were occasional synaptophysin positive, suggesting neuroblastic differentiation. Post-operative computerized tomography (CT) showed radical excision of lesion [Figure 2]. She received post-operative radiotherapy of 54 Gy in 30 fractions and 2 cycles of chemotherapy (etoposide and cisplatin). 8 months later, she presented with features of increased intracranial pressure (ICP) with history of headache, multiple episodes of seizures and an altered sensorium. Fundus examination revealed papilloedema. Repeat imaging revealed large irregularly, enhancing recurrent lesion with central cystic and hemorrhagic components in right temporo-parietal region with perilesional edema, causing mass effect and midline shift [Figure 3]. Imaging also showed multiple small enhancing lesions in the periventricular region around the frontal and occipital horn of the other side. She underwent right temporo-parietal re-craniotomy and gross total excision of the lesion. Post-operatively, she improved in her neurological status. Histopathological examination confirmed a glioblastoma multiforme with high mitotic index and radiation induced changes. Histopathology also revealed that GBM had primitive neuro-ectodermal tumor (PNET) like differentiation, similar to the previous HPE. In the post-operative period, the patient was neurologically stable with no new neurological deficits. Post-operative CT, done on 6th post-operative day, showed radical excision with a small amount of residual tumor in the right parietal region [Figure 4]. To our surprise, the periventricular lesion around the left occipital region had grown very rapidly [Figure 4c]. Even the frontal lesion had increased in size, and new lesion was seen around the 4th ventricle [Figure 4a]. Poor prognosis was explained to the patient's attendants, and the patient was discharged. She continued to have seizures 2 weeks after the surgery. 6 weeks after the surgery, the child developed multiple seizures, followed by decreased conscious level and expired.
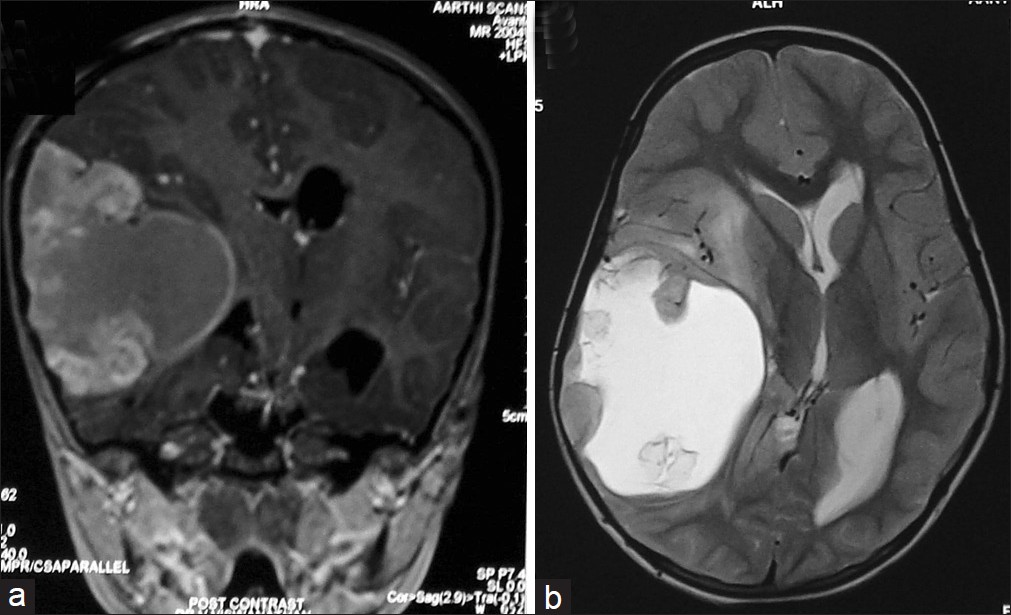
- MRI of the brain (a) T1 coronal section (b) T2 axial section – showing mixed intensity contrast enhancing lesion which is predominantly cystic

- Computerized tomography scan of the brain contrast study after the first surgery showing radical excision of the lesion.
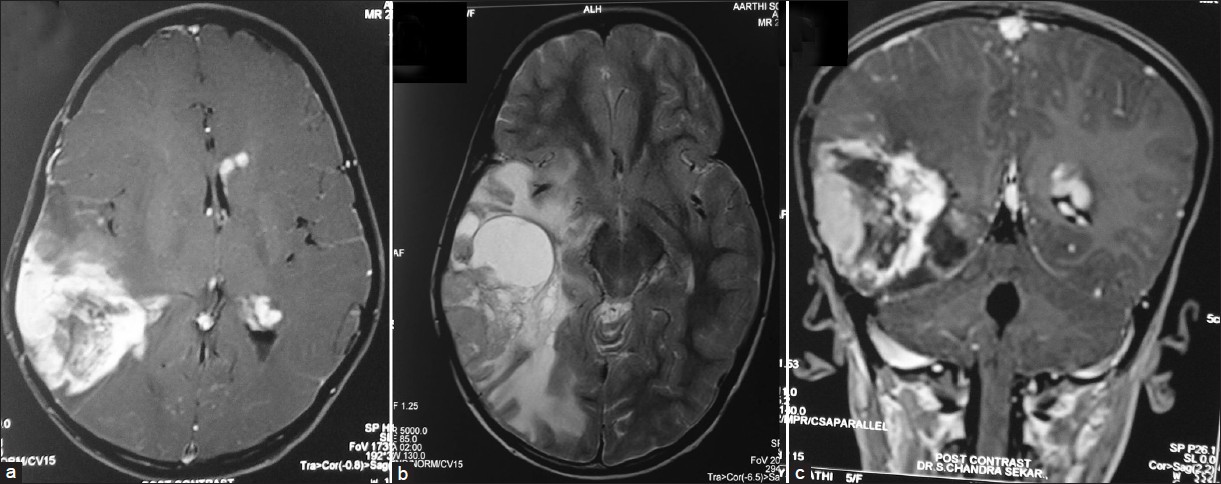
- (a-c) Contrast magnetic resonance imaging of the brain showing large irregularly enhancing recurrent lesion with central cystic and hemorrhagic components in right temporo-parietal region with perilesional edema causing mass effect and midline shift. Small enhancing lesions in the periventricular regions around the frontal and occipital horn on the left side can be seen in a and b

- (a-c) Post operative contrast computerized tomography scan of the brain showing radical excision of the right temporo parietal lesion with a small amount of residual in the right parietal region (c). Periventricular lesions around the left occipital and frontal region are increased in size compared to previous scan (c). New lesion is seen around the fourth ventricle (a)
Discussion
Glioblastoma multiforme is the most common primary malignant brain tumor. However, non-brain stem glioblastoma in children is less common than in adults. The outcome of pediatric patients operated for GBM is better than adults.[1–3] There are many studies, which support this statement, and there are reports of pediatric patients with GBM who are alive even after 24 years.[1] All the studies emphasize on gross total resection as the most important factor for achieving a good outcome.[1]
In 1963, Batzdorf and Malamud have defined a criterion that permits to distinguish multifocal and multicentric tumors.[4] The multifocal tumor is a result of (i) dissemination or growth by an established route like, spread via commissural or other pathways (corpus callosum, fornix, internal capsule or massa intermediate), (ii) spread via cerebrospinal fluid channel, (iii) local metastasis. Multicentric gliomas are widely separated lesions in different lobes or hemisphere that cannot be attributed to the pathways mentioned above.[5] Multicentric gliomas can be mistaken for metastatic deposits.[6] The pattern of development of a multifocal GBM can be of 2 types; synchronous or metachronous. In this reported case, subsequent lesions developed metachronously, 8 months after diagnosing the primary lesion. The location of the lesions, which developed subsequently, is around the ventricle; hence, cerebrospinal fluid (CSF) dissemination may be the probable cause of the spread in the present case. Radiation induced intracranial malignancies is well-known entity. In the present case, as the lesions appeared after radiotherapy, role of radiation in the development of multifocal GBM is also to be considered. However, there are no studies confirming the ‘cause and effect’ relation between the two.
Genetic analysis of patients with multifocal GBM have shown that homozygous deletion of the p16 locus, inactivation of the phosphatase and tensin homolog (PTEN) gene by loss of heterozygosity, a balanced translocation [t(1;15)(p3?6;q2?5)] and p53 mutation may play a role, either collectively or independently.-[7] Showalter et al have reported the mean age of presentation of multifocal GBM as 61 years.[78] Multifocal GBM in pediatric age group is rarely reported. The pediatric variant of GBM has a better outcome compared to the adults.[1239] Song et al have reported that 1 and 5 year survival of pediatric GBM in their study was 67% and 40%, respectively. Patients with complete resection had a significantly longer overall survival and progression free survival as compared to patients with an incomplete resection.[2] Patients with MIB-1 labeling index higher than 36% had a poor outcome.[9] More studies are required to ascertain whether the same holds good for multifocal GBM. The management and outcome of pediatric multifocal GBM is not studied completely. The limitation of such studies is the rarity of this entity. In the present case, as there were multiple lesions and they were rapidly growing, further surgical intervention had no role to either improve the quality of life or to increase the survival. As the child had received radiotherapy only 8 months back, booster dose of radiotherapy was not a feasible option. The role of adjuvant chemotherapy in such cases is also of doubtful effect. In the author's opinion, achieving a gross total resection in GBM may reduce the chances of multifocal GBM. In metachronously developed multifocal GBM, repeated resections can be offered if there is a chance to improve the quality of life. Concomitant chemo-radiation with fractionated radiotherapy and temozolamide has been advocated to get better results. In cases where there is high mitosis or high MIB index, resulting in a very rapid rate of tumor growth; decision regarding further treatment should be taken judiciously. The line of treatment can be justified if it positively influences one or more of the following: Improving the neurological deficits, quality of life, progression free survival, overall survival and retarding disease progression. With an advancement in neuroimaging, large numbers of such cases are being recognized either at diagnosis or at follow-up. Further studies in larger group of patients will throw more light on the outcome and prognosis of these rare tumors. Majority of patients with malignancy receive cancer therapy as participants in clinical research protocols, participation in oncology trials has become the “standard of care” in pediatric oncology.[10] There are a few phase I trials being conducted for high grade gliomas. In one of the phase I trial, the safety of lenalidomide is being tested.[11] High grade gliomas are included in ongoing phase II cancer chemotherapy trials at various centers.[12] In patients with genetic predisposition where there is expression / deletion of certain genes like PTEN, P53 and others, there are phase I and II trials being conducted for such specific subpopulation[13] In such rare entities, there are not many ongoing phase III trials. Hence, such rare tumors can be referred for ongoing phase I or phase II trials.
Conclusion
Pediatric variants of GBM also show multifocal tumor development. Multifocal GBM results from dissemination or growth by an established route of spread via commissural or other pathways or spread via cerebrospinal fluid channel or local metastasis. In metachronously developed multifocal GBM, repeated resections can be offered if there is a chance to improve the quality of life. More studies are required to study the behavior, management and outcome of these rare tumors.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Glioblastoma in Children: A Single-Institution Experience. Int J Radiat Oncol Biol Phys. 2011;80:1117-21.
- [Google Scholar]
- Long-term outcomes in children with glioblastoma. J Neurosurg Pediatr. 2010;6:145-9.
- [Google Scholar]
- Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227-35.
- [Google Scholar]
- CT findings in multicentric glioblastoma: diagnostic-pathologic correlation. J Comput Tomogr. 1980;4:187-92.
- [Google Scholar]
- Multicentric and isolated multifocal glioblastoma multiforme simulating metastatic disease. Br J Radiol. 1975;48:10-5.
- [Google Scholar]
- Multifocal glioblastoma multiforme: Prognostic factors and patterns of progression. Int J Radiat Oncol Biol Phys. 2007;69:820-4.
- [Google Scholar]
- Genetic analysis of a multifocal glioblastoma multiforme: A suitable tool to gain new aspects in glioma development. Neurosurg. 2003;53:1377-84. discussion 1384
- [Google Scholar]
- Impact of proliferation index on outcome in childhood malignant gliomas: Results in a multi-institution-al cohort. Neurosurg. 2002;50:1238-45.
- [Google Scholar]
- Bethesdatrials.cancer.gov. National Cancer Institute. Available from: http://bethesdatrials.cancer.gov/pediatric/index.aspx
- [Google Scholar]
- Pediatric phase II cancer chemotherapy trials: A Pediatric Oncology Group study. J Pediatr Hematol Oncol. 1997;19:187-91.
- [Google Scholar]
- A pilot study to assess the tolerability and efficacy of RAD-001 (everolimus) with gefitinib in patients with recurrent glioblastoma multiforme. Neuro-Oncology. 2006;8:447.
- [Google Scholar]






