Translate this page into:
Pediatric brainstem oligodendroglioma
Address for correspondence: Dr. Sandeep Mohindra, Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India. E-mail: sandeepneuro@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The authors present the first report of pediatric brainstem oligodendroglioma, infiltrating midbrain, and medulla oblongata. The report details clinical features, radiological findings, and surgical steps. As this entity is exceedingly uncommon, the overall epidemiology, prognosis, and long-term outcome remain far from established.
Keywords
Brain stem tumors
Oligodendroglioma
Pediatrics
Introduction
Oligodendroglioma (ODG) accounts for 1-2% of all primary intracranial tumors among children.[12] The majority of ODGs are single lesions, located in the cerebral hemispheres, involving subcortical white matter and the overlying cortex. Infratentorial ODG is a rare occurrence. Brainstem remains the rarest site of ODG occurrence.[3–6] English literature describes six cases of intrinsic brainstem ODGs and 10 cases of exophytic brainstem ODGs.[1–6] The present report describes a child with brainstem ODG who presented with obstructive hydrocephalus, on account of a large pontine mass lesion, infiltrating midbrain, and medulla oblongata.
Case Report
A 13-year-old girl presented with complaints of bifrontal headache, associated with projectile vomiting for 6 months duration. In the last fortnight, she had developed visual obscurations and ataxic gait. She had deviation of angle of mouth to the right side while talking and smiling for 2 months. In the last 5 days, she had developed drooping of both eyelids. On examination, the child was conscious, with intact cognition. She had dysarthric speech with a nasal twang. She could count fingers at 2 meter with the right eye, while mere perception of light with the left eye. Bilateral ptosis was present, more prominent on the left side. The left eye was deviated laterally and superiorly. Left-sided lower motor neuron palsy of the seventh cranial nerve was present. Bilateral gag reflex was weak. She had spastic quadriparesis. Thus, signs and symptoms were suggestive of bilateral, multiple, asymmetrical cranial nerve palsies with long tract signs consistent with intrinsic brainstem lesion, localized to the pons and midbrain, more towards the left side.
Computed tomography (CT) scan of the head revealed mass lesion in the brainstem, compressing and obscuring the fourth ventricle, leading to obstructive hydrocephalus. After right-sided ventriculo-peritoneal (VP) shunt, the patient underwent contrast-enhanced magnetic resonance imaging (MRI) study, which showed a large pontine mass lesion, protruding from the floor of the fourth ventricle and occluding it. The mass lesion was hypo-intense on T1-weighted scan and iso-intense to hyper-intense on T2 scan. On contrast administration, there was variegated contrast enhancement, with well delineation of cystic regions within the tumor [Figure 1a and b]. With a clinico-radiological diagnosis of pediatric brainstem glioma, the patient underwent midline sub-occipital craniectomy and near-total excision of tumor. Intraoperatively, the median sulcus was split and tumor was entered, which was debulked using cavitron ultrasonic suction aspirator (CUSA). The tumor was soft, friable, with intermittent regions of fluid-filled cavities, suggestive of old hemorrhages and necrosis. The tumor was mildly vascular. Postoperatively, the patient's deficits remained static, except for increased right-sided motor weakness. The patient was discharged on eighth postoperative day and was subjected to craniospinal irradiation of 50 Gy. Histopathology showed moderately cellular neoplasm with a monotonous pattern of uniformly rounded hyperchromatic nuclei separated by delicate branching network of capillaries. Further, higher magnification showed psammoma body like micro-calcification in the tumor. Overall features were suggestive of oligodendroglioma [Figure 1c]. At 1 year of follow-up, the child is ambulatory with a Karnofsky Performance Scale (KPS) of 90%. The diplopia has disappeared and there is no ptosis. MRI shows satisfactory tumor excision and tumor control. There is no evidence of recurrence of tumor at the operative site or spinal cord. Further, immunohistochemistry showed positive glial fibrillary acidic protein (GFAP) immunostain in the glial component. Nuclear Ki67 labeling in the tumor cells was positive [Figure 1d].
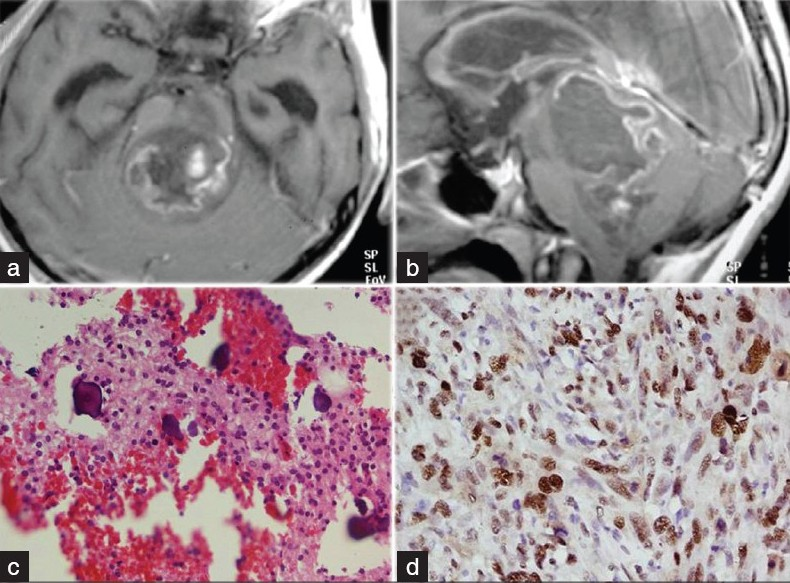
- (a) T1-weighted contrast-enhanced image showing variegated contrast uptake by the tumor. (b) Sagittal section showing pontine tumor completely occluding the fourth ventricle. (c) Photomicrograph showing psammoma body like micro-calcification in tumor (Hematoxylin and Eosin, ×200). (d) Photomicrograph showing nuclear Ki67 labeling in the tumor cells (Ki67 immunostain, ×400).
Discussion
In children, brainstem gliomas account for 10-30% of posterior fossa tumors, but only a few of these are ODG in nature.[1–6] The incidence of true brainstem ODG has not been well studied, likely because of their rarity. In the past era, because of non-availability of high-end imaging tools (MRI scans), the true incidence of these brainstem ODGs cannot be determined.[3]
Median age for these brainstem ODGs is 11.5 years, which is significantly less as compared to patients harboring supratentorial ODGs (median age, 25-40 years).[3] Most brainstem ODGs are located in the pons and/or medulla, while one case of ODG located in the cervico-medullary region has been reported.[3] Invasion of the midbrain was observed only in instances of tumor spread from cerebellar ODG.[7]
The present case is unique where invasion of the midbrain from pontine tegmentum is described. The well-maintained arachnoid layer over the floor of fourth ventricle indicated the containment of tumor within the brainstem and explained the cranial spread of tumor to invade the midbrain. This observation is in contrast with previously described cases in the literature.[3] These ODGs have a distinct propensity to infiltrate along white matter tracts of the posterior fossa, which is revealed by the preferential involvement of left-sided dorsal columns of the mid brain. The infiltrative capabilities of ODGs and their propensity to disseminate via the cerebrospinal fluid (CSF) are also now well established.[8] Relatively short clinical history for this group of ODGs is attributed to limited availability of space in the posterior fossa, combined with dense apposition of vital nuclei and tracts.[23] ODGs of posterior fossa are frequently cystic, with calcifications.[9] The aggressiveness of these tumors may not be in accordance with these parameters. The degree of cellular atypia, presence of vascular proliferation, necrosis and mitotic figures are better prognostic factors. Henceforth, aggressive biology combined with early presentation mark poor prognosis for such patients.[1–3] Rare cases with good long-term follow-up and/or survival are on records.[3] The present case remains disease-free at 12 months of follow-up.
Conclusion
Brainstem ODGs may be considered pathological surprises, as the rarity of these lesions forbids defining clear radiological criteria for them. Pontine tegmentum provides the pathway of tumor spread from pons to the mid brain. Radical extirpation of the tumor from such an eloquent part of the brain is possible and should be attempted. Cranio-spinal irradiation remains the adjuvant treatment of choice and a long-term outcome may not be nihilistic as previously believed.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Primary intrinsic brainstem oligodendroglioma in an adult. Case report and review of the literature. J Neurosurg. 1996;85:1165-9.
- [Google Scholar]
- Stereotaxic suboccipital transcerebellar biopsy of pontine mass lesions. J Neurosurg. 1989;70:195-200.
- [Google Scholar]
- Analysis of a surgical series of 49 oligodendrogliomas with 3 infratentorial localizations. Neurochirurgie. 1967;13:679-700.
- [Google Scholar]
- Oligodendrogliomas arising from structures of the posterior fossa. Neurology. 1952;2:461-70.
- [Google Scholar]
- Oligodendrogliomas of the fourth ventricle: Report of two cases. J Neurol Neurosurg Psychiatry. 1969;32:226-9.
- [Google Scholar]
- Infratentorial oligodendrogliomas: Imaging findings in six patients. Acta Radiol. 2010;51:213-7.
- [Google Scholar]
- Low-grade oligodendroglioma of the pineal gland: A case report and review of the literature. Diagn Pathol. 2010;5:59.
- [Google Scholar]






