Translate this page into:
Pathophysiology of acute middle cerebral artery infarct by multimodal computed tomography: A pilot study in Thai patients
Address for correspondence: Dr. Pornpatr A. Dharmasaroja, Division of Neurology, Department of Internal Medicine, Thammasat University, Klong 1, Klong Luang, Pathumthani 12120, Thailand. E-mail: pornpatr1@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aims:
The purpose of this study was to study pathophysiology of acute middle cerebral artery infarct using multimodal CT and to evaluate the safety and feasibility of this method in our center.
Materials and Methods:
Patients who had moderate to severe stroke (NIHSS score > 10), suspected of anterior circulation infarct and presented within 4 hours after stroke onset were prospectively included. Multimodal CTs, using low-osmolar contrast agents, were performed in all patients.
Results:
Twenty-two patients were included. Mean NIHSS was 16. All patients received intravenous thrombolysis. Favorable outcome was found in nine patients (41%). CTP was unable to identify ischemic lesions in three patients with small subcortical infarct. Most patients (82%) with large middle cerebral artery infarct still had some salvageable brain (penumbra) which partly recovered in a follow-up imaging. Eleven patients (50%) had major artery occlusion. Two patients had creatinine rising within 72 hours.
Conclusions:
Multimodal CT does provide information about status of major artery and the volume of salvageable/infarct brain tissue and is safely and easily applicable in our center.
Keywords
Computed tomography angiography
computed tomography perfusion
multimodal computed tomography
stroke
Introduction
Stroke is one of the leading causes of death and morbidity in Thailand. Patients with more severe stroke have higher mortality and more residual deficits in the survivals. Although new treatments have been developed to rescue the ischemic brain during acute stroke, this seems to be less effective in patients with severe stroke. Patients with high National Institute of Health Stroke Scale (NIHSS) scores as compared to those with less severe stroke, usually have poorer outcome, higher rates of mortality and symptomatic intracerebral hemorrhage even after intravenous recombinant tissue plasminogen activator (rtPA) treatment.[1234] Patients with a NIHSS score of 10 or more have a greater than 80% likelihood of a major arterial occlusion.[56] Endovascular treatments provide more recanalizations in the occluded arteries, but the favorable outcomes of the treated patients do not significantly differ from those with intravenous thrombolytic treatment.[7] The hypothesis regarding penumbral imaging selection was that some patients have substantial regions of salvageable brain tissue during the treatment while others may not.[8]
Multimodal computed tomography, including non-contrast computed tomography (CT), computed tomography perfusion (CTP) and computed tomography angiography (CTA), has been increasingly used in research and clinical practice. CTP provides quantitative data about cerebral blood flow and CTA reveals the patency of the arteries. Application of multimodal CT may provide important information which helps to make decision about the appropriate choice of acute treatment and also the prognosis of the patients. In Thailand, this technique is new, and whether it can be safely and easily applied to our patients is not known. The purpose of this study was to study pathophysiology of acute middle cerebral artery infarct using multimodal CT and evaluate the safety and feasibility of this method in our center.
Materials and Methods
All eligible patients with acute ischemic stroke treated at Thammasat University Hospital between January 2013 and December 2013 were included. Eligible criteria were presentation within 4 hours after stroke onset, moderate to severe neurological deficits (an NIHSS score > 10), suspected anterior circulation infarct and no previous history of renal failure. Informed consent was obtained from the patients or a legal representative before enrollment. The study was approved by the institute's ethical review committee. Information about baseline characteristics of the patients, cardiovascular risk factors, stroke subtypes, severity of stroke and baseline creatinine was collected. Stroke severity was evaluated by the NIHSS.
Multimodal CT was performed in all included patients, using a 64-slice CT scanner (Philips Brilliance 64) in the first 10 patients and 256-slice CT (Philips Brilliance ICT) in the rest. Sixteen contiguous slices of 5-mm thickness (for a total length of 80 mm) and 24 slices of 5-mm thickness (a total length of 120 mm) were covered in 64-slice CT and 256-slice CT, respectively. Non-ionic, low-osmolar contrast agent of 50 ml was used for CTP and another 50 ml for CTA by 64-slice CT. For the 256-slice CT, the contrast was used at lower doses: 35 ml for CTP and 35 ml for CTA. Color-coded perfusion maps was calculated out of the source of data (cerebral blood volume (CBV) and mean transit time (MTT)), deconvolved and thresholded. The ischemic tissue (penumbra) shows increased MTT with decreased cerebral blood flow (CBF) and normal or mildly increased CBV, whereas infarct tissue shows markedly decreased CBF, CBV and increased MTT.[9] The CTP map program used a threshold for core infarct when CBV is less than 2 mL/100 gm of tissue and for ischemic tissue when MTT is over 150%. Time spent in performing multimodal CT was recorded. All patients received intravenous 0.9% normal saline at the rate of at least 1 ml/kg/hour for the first 24 hours. Patients with no contraindication by standard exclusion criteria were treated with intravenous rtPA. Another CT or magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) was performed after rtPA treatment. MRAs were used to assess recanalization. All patients have a follow-up creatinine within 72 hours after multimodal CT. Contrast-induced nephropathy (CIN) is defined as a 25% or more increase in baseline creatinine levels within 72 hours of contrast administration.[10] Incidence of CIN was collected.
The Modified Rankin scale (mRS) was used to assess patient outcomes 3 months after the onset of stroke. A favorable outcome was defined as a mRS score of 0 or 1. The radiological definition of hemorrhagic events followed the ECASS classification: (i) HI-1 = hemorrhagic infarction type 1 (small petechiae along the margins of the infarct); (ii) HI-2 = hemorrhagic infarction type 2 (confluent petechiae within the infracted area, but without space-occupying effect); (iii) PH-1 = parenchymal hematoma type 1 (a hematoma in < 30% of the infarcted area with slight space-occupying effect); and (iv) PH-2 = parenchymal hematoma type 2 (a dense hematoma > 30% of the infarcted area with substantial space-occupying effect, or any hemorrhagic lesion outside the infarcted area).[11] A hemorrhage was considered symptomatic (according to NINDS study criteria) if it had not been identified on a previous CT scan and there had subsequently been either a suspicion of hemorrhage or any decline (NIHSS > 1) in the neurological status of the patient. Ischemic brain volume was compared between patients with and without artery occlusion or severe focal stenosis using Student's t-test (for continuous variables).
Results
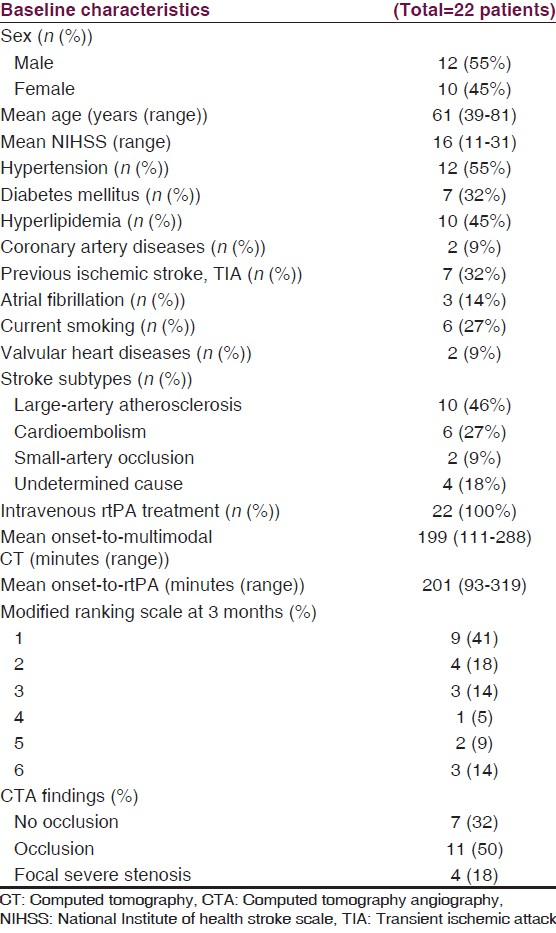
There were 22 patients included during the study period. Baseline characteristics of the patients are presented in Table 1. All patients received intravenous rtPA treatment. Most patients (82%, 18 out of 22 patients) with middle cerebral infarct who presented within 4 hours had mismatch or still had some salvageable brain (penumbra) which partly recovered in a follow-up imaging [Table 2, Figure 1 patient number 10]. Nine patients (41%) in our study had favorable outcome at 3 months. Asymptomatic intracerebral hemorrhage was found in 11 patients. Three patients died within 3 months because of sepsis. Mean creatinine level at baseline was 1.06 (range from 0.6 to 2). Mean fluid intake, mainly via intravenous route, during the first day of admission was 1655 ml. Most patients had slightly decreased or stable creatinine level within 72 hours, but 2 patients, one with early septic shock and another with early brain edema and having mannitol treatment, had increased creatinine level from the baseline.


- Patient No. 10; CT perfusion showed mismatch in the right middle cerebral artery distribution. Patient No.1: CT perfusion revealed unremarkable, while follow-up diffusion-weighted image (DWI) showed acute infarct at right periventricular area. Patient No.20; CT perfusion showed slightly increased CBV at right temporal area without prolonged MTT, which may be explained by early reperfusion. Follow-up DWI revealed acute infarct at right temporal lobe
Mean duration of performing multimodal CT was 12 minutes (range from 5-20 minutes). CTP was unable to identify penumbra and infarct core in three patients, while a follow-up imaging showed infarct at a subcortical area (putamen and extended to periventricular area) [Figure 1; patient number 1]. CTP underestimated an ischemic lesion (right temporal lobe) in one patient who had early reperfusion at the infarct lesions [Figure 1; patient number 20]. CTA revealed occlusion in intra/extracranial arteries in 11 patients (50%), focal severe stenosis in 4 patients (18%) and no occlusion in 7 patients (32%). Patients with occlusion or severe focal stenosis had larger penumbra (91 ml vs 61 ml, P = 0.36), core infarct (69 ml vs 10 ml, P = 0.14) from CTP and infarct volume (122 ml vs 27 ml, P = 0.08) from the follow-up imaging, as compared to patients with no occlusion. Recanalization was found in 60% of the patients with occlusion or severe intracranial stenosis.
Discussion
With the CTP map, it is easy to estimate the salvageable, and infarct brain tissue, just after the perfusion scan is finished. The diagnostic accuracy of CTP for detecting acute ischemic stroke was evaluated in a systemic review, including 15 studies (1,107 patients).[12] A pooled analysis resulted in a sensitivity of 80% and a specificity of 95%. Almost two thirds of the false negatives were due to small infarcts, and the remaining false negatives were mostly due to limited coverage. CTP could not identify ischemic lesions in three patients in our study. All the missed lesions were small and located in the subcortical area.
From a previous study, patients with a NIHSS score of 10 or more have a greater than 80% likelihood of a major arterial occlusion.[56] In our study, 50% of the patients with NIHSS > 10 had major arterial occlusions and almost one fifth of the patients had focal severe stenosis in major arteries that cause acute brain ischemia. However, about a third of the patients had no occlusion in major arteries, but some of them still had penumbra. Patients without occlusion of major arteries tended to have smaller penumbra and final infarct volume as compared to patients with severe stenosis/occlusion in major arteries. The difference was not statistically significant, which may be due to the small number of patients in our study.
Patients who have more severe neurological deficits, older age and persistent arterial occlusion are most likely to have unfavorable clinical outcome.[13] Intravenous thrombolysis achieves partial or complete recanalization in only 30% to 50% of patients, but lower recanalization rates in proximal large-vessel occlusions. In angiographic trials of intravenous thrombolysis, partial or complete recanalization was achieved in only 10% of occluded internal carotid arteries, and in 25% of proximal middle cerebral arteries.[14] Endovascular treatment provide higher rates of recanalization (46%-88%), but only modest rates of good outcomes (25%-54%) were observed in those patients. This may, in part, be due to the treatments of patients who have already large infarcts or those who do not have a clinically relevant volume of salvageable brain tissue.[8] A previous study showed that infarct volume on admission CTP correlated with final infarct volume and clinical outcome. For those presenting with a CTP infarct core > 100 ml, the predictive value for a poor clinical outcome was 100%.[15] Severity of stroke is related to symptomatic intracerebral hemorrhage after intravenous rtPA treatment. Thus, a combination of CTP and CTA will provide important information before treatment, especially in patients with acute severe stroke. The decision for appropriate choice of treatment can be made if the doctors know the location of the occlusions and whether the patients still have a substantial amount of salvageable brain tissue to save.
This pilot study showed that multimodal CT was safe and feasible. Our study revealed that it took approximately 12 minutes to complete multimodal CT studies. Running scan time in the machine was just 2 minutes for CTP and CTA. In the settings of acute stroke, CT needs to be done as soon as possible to provide the information needed to immediately make a treatment plan. Some patients were unable to communicate and it was not feasible to use the usual recommended treatment to prevent the development of CIN. CIN was a particular concern. Many stroke patients were old and had several vascular risk factors, including chronic kidney disease. The important risk factors of CIN were history of chronic kidney disease, diabetes mellitus and type of contrast agents used.[16] Nonionic low-osmolar contrast agents were associated with lower risk of CIN than high-osmolar contrast agents. On the question of dose of contrast agent, it is still debated whether higher volume is linked to CIN. Hopyan et al. evaluated renal safety of CTA and CTP imaging, using lower-risk contrast agents (low-osmolar and iso-osmolar agents), in 198 patients with acute stroke.[16] Although the incidence of contrast-induced nephropathy (CIN) occurred in 2% of the patients, none developed chronic kidney disease or required dialysis. The authors concluded that when patients without an available serum creatinine are screened for a history of chronic kidney disease and patients with advanced kidney disease (glomerular filtration rate (GFR) < 30 ml/min) are excluded, the chance of developing long-term renal sequelae is quite low.
In our study, multimodal CT was performed in the first 10 patients, using 64-slice CT scanner (total 100 ml of low-osmolar contrast agent for CTA and CTP) and the 12 subsequent patients, with 256-slice CT (70 ml of contrast). There was no difference in the incidence of CIN between using 100 ml and 70 ml contrast agents. Two patients had increased creatinine level from the baseline, meeting the criteria of CIN. However, both patients had other causes that precipitated acute kidney injury. One patient had early septic shock. The other patient had early brain edema and required mannitol treatment, which caused dehydration and worsened the kidney injury. This patient had decompressive surgery on day 2 after admission.
One of the limitations of our study was the small number of patients. Slow recruitment may be due to the strict inclusion criteria. Only patients with severe stroke, suspected of middle cerebral artery infarct and presented within 4 hours after stroke onset were enrolled. Another limitation was using different CT scanners within this cohort. Lower volume of contrast was used and smaller area of brain imaging was covered with 64-slice CT scanner as compared to 256-slice CT scanner, which might cause a small difference in results of volume of penumbra and core infarct.
In conclusion, performing multimodal CT was safely and easily applicable in our center and provided useful information about status of major arteries and the volume of salvageable/infarct brain tissue. Although the cost of multimodal CT is still expensive in Thailand, selected patients with suspected of suffering major artery occlusion will be benefited from the procedure.
Source of Support: Faculty of Medicine, Thammasat University.
Conflict of Interest: None declared.
References
- Outcomes of Thai patients with acute ischemic stroke after intravenous thrombolysis. J Neurol Sci. 2011;300:74-7.
- [Google Scholar]
- Safe Implementation of Thrombolysis in troke-Monitoring Study Investigators. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST) Stroke. 2008;39:3316-22.
- [Google Scholar]
- Intracerebral hemorrhage following intravenous thrombolysis in Thai patients with acute ischemic stroke. J Clin Neurosci. 2012;19:799-803.
- [Google Scholar]
- SAINT Investigators. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: Pooled analysis of placebo data from Stroke-Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke. 2009;40:3067-72.
- [Google Scholar]
- NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36:2121-5.
- [Google Scholar]
- Relationships between angiographic findings and National Institutes of Health stroke scale score in cases of hyperacute carotid ischemic stroke. AJNR Am J Neuroradiol. 2004;25:238-41.
- [Google Scholar]
- Interventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903.
- [Google Scholar]
- MR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914-23.
- [Google Scholar]
- CT protocol for acute stroke: Tips and tricks for general radiologists. R adiographics. 2008;28:1673-87.
- [Google Scholar]
- Canadian Association of Radiologists. Canadian Association of Radiologists: Consensus guidelines for the prevention of contrast-induced nephropathy. Can Assoc Radiol J. 2007;58:79-87.
- [Google Scholar]
- Randomiseddouble-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245-51.
- [Google Scholar]
- Diagnostic accuracy of CT perfusion imaging for detecting acute ischemic stroke: A systemic review and meta-analysis. Cerebrovasc Dis. 2013;35:493-501.
- [Google Scholar]
- Reperfusion therapies for acute ischemic stroke. Curr Treat Options Neurol. 2010;12:155-66.
- [Google Scholar]
- Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol. 1993;14:3-13.
- [Google Scholar]
- Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: Prediction of final infarct volume and clinical outcome. Stroke. 2001;32:2021-8.
- [Google Scholar]
- Renal safety of CT angiography and perfusion imaging in the emergency evaluation of acute stroke. AJNR Am J Neuroradiol. 2008;29:1826-30.
- [Google Scholar]






