Translate this page into:
Optochiasmatic involvement in tuberculous meningitis: A case series with a review of literature
*Corresponding author: Krishnan Nagarajan, Department of Radio-Diagnosis, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. lknagarajan1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sriviruthi B, Amuthabharathi M, Nagarajan K, Ramesh Babu K, Biswal N, Vivekanandan M. Optochiasmatic involvement in tuberculous meningitis: A case series with a review of literature. J Neurosci Rural Pract. doi: 10.25259/JNRP_36_2025
Abstract
Optochiasmatic tuberculosis (TB) is a rare but potentially debilitating manifestation of central nervous system TB. This form of TB, which involves the inflammation of the optic chiasm and surrounding structures, can lead to profound vision loss and significant neurological impairment if not promptly diagnosed and treated. In this case series, we have reviewed the clinical presentation, visual assessment, imaging findings, cerebrospinal fluid analysis, and follow-up of six patients diagnosed with optochiasmatic TB, managed at a tertiary care center in South India. The time from starting antituberculous treatment (ATT) to the onset of visual symptoms varied, with a mean interval of 5.67 (±2.05) months. Visual impairment was noted in 50% of the cases at presentation. All patients underwent contrast-enhanced magnetic resonance imaging, which revealed characteristic findings of tuberculomas and exudative changes around the optic chiasm. All six patients received ATT, corticosteroids, and symptomatic management. Follow-up imaging of three patients (50%) showed a significant reduction in tuberculoma size, lesion number, and edema, indicating a favorable response to treatment.
Keywords
Magnetic resonance imaging
Optochiasmatic arachnoiditis
Tuberculosis
Tuberculous meningitis
Vision loss
INTRODUCTION
Optochiasmatic involvement in tuberculosis (TB) can manifest as either optochiasmatic arachnoiditis (OCA) of the leptomeninges around the chiasma or tuberculoma, both of which are vision-debilitating forms of TB meningitis (TBM). It accounts for about 18% of cases of TBM and can result in significant vision loss and/or cranial nerve damage.[1] Optochiasmatic involvement refers to the inflammation of the pia-arachnoid mater of the optic nerve and chiasma in the chiasmatic and suprasellar cisterns. It is typically severe exudative meningitis, characterized by large amounts of inflammatory exudate in the optic chiasm and suprasellar cisterns. Visual loss in these cases can be due to the direct mechanical compressive effect on the anterior optic pathway – optic nerve and chiasma – or, ischemia or both. Involvement of the vasa vasorum supplying these nerves as vasculitis can also result in visual deficits.[2] Early diagnosis and prompt initiation of treatment are critical in preventing vision loss.
CASE SERIES
We report six cases of tuberculous meningitis with optochiasmatic involvement managed at a tertiary care institution in south India. All patients underwent contrast-enhanced magnetic resonance imaging (CE-MRI) as per institutional protocol. Cerebrospinal fluid (CSF) analysis and visual assessment were done on admission. Ophthalmology evaluation included extraocular movement assessment for cranial nerve palsies and swinging flashlight test for pupillary light reflex. The corrected visual acuity was assessed along with a fundus examination. The clinical presentations, neuroimaging findings, CSF analysis, and follow-up of these cases are summarized in Table 1.
| S.No. | Age/Sex | History | EOM assessment and visual examination | MRI findings | CSF analysis | Follow-up |
|---|---|---|---|---|---|---|
| 1 | 20/F | Known TB meningitis, post 6 months of ATT and VP shunt for hydrocephalus 9 months back. Now developed headache, fever, and gradual vision loss |
Bilateral 6thcranial nerve palsy; VA: Both eye-perception of light; Pupils: Dilated and fixed; (WHO) Fundus: Normal; VP: Not stimulable |
- Few RELs in suprasellar- chiasmatic cistern (largest 13 x 7 mm) - RELs in right parietal and anterior temporal regions |
Protein 270 mg/dL; Glucose 43 mg/dL; WBC 300 cells/mL (N: 30, L: 70) |
Reduction in tuberculoma size (13x 7 mm to 7 x 7 mm on 3 years follow-up) |
| 2 | 11/F | Known TB meningitis, on 3 months of ATT, VP shunt done for hydrocephalus. Now has headache, seizures, diplopia, and right eye ptosis |
Right 3rdcranial nerve palsy; VA: Right eye -no perception of light; Left eye - 6/18; Right pupil: Dilated and fixed; Fundus: Normal |
- Multiple conglomerate RELs in hypothalamus, inferior third ventricle, optic chiasma, interpeduncular and suprasellar cisterns - RELs with perilesional edema in right cerebral peduncle and midbrain |
- | Reduction in size and number of lesions, reduction in edema on 1 year 3 months follow-up Mild cerebellar atrophy (phenytoin-induced) |
| 3 | 24/F | Known CNS tuberculosis, left cerebellar tuberculoma – operated & VP shunt done for hydrocephalus, on ATT since 8 months. Now pregnant (6 months) developed a fever, headache, and blurring of vision. Presented in altered sensorium. |
VA: 6/60 both eyes; Pupils: Reactive to light, anisocoria; Fundus: Normal. |
- Coalescent RELs in suprasellar cistern with perilesional edema - Chronic infarcts in right thalamus and left caudate nucleus - Focal gliosis in left inferior cerebellar hemisphere and tonsil |
Protein 292 mg/dL; Glucose 22 mg/dL |
Termination of pregnancy, reduction in size and number of lesions, reduction in edema on 1 year 4 months follow-up, on continued 8 years follow-up only small residual seen Left peritrigonal gliosis |
| 4 | 36/F | Known retroviral and hepatitis-B infection Headache, gradual blurring of vision, seizure |
VA: Left eye - counting fingers at 3 meters; Right eye - 6/60. Bilateral pupil: Reactive to light; Fundus: Normal. |
- Leptomeningeal enhancement with multiple conglomerate RELs in optic chiasm, anterior commissure, and pituitary infundibulum - RELs in anterior interhemispheric fissure, bilateral cerebral and cerebellar hemispheres, midbrain tectum - Acute infarct in left basal ganglia - Mild hydrocephalus |
Protein 205 mg/dL; Glucose 15 mg/dL; WBC 140 cells/mL (N: 20, L: 80) |
No follow-up imaging |
| 5 | 6/F | Fever, vomiting, no neurological deficits or visual complaints | VA: 6/6 both eyes; Bilateral pupils: Reactive to light; Fundus: Normal. |
- Leptomeningeal enhancement in Sylvian, suprasellar and interpeduncular cisterns - Multiple conglomerate RELs in supra-optic recess and hypothalamus - Few RELs in right frontal lobe |
Protein 116 mg/dL; Glucose 21 mg/dL; WBC 220 cells/mL (N: 20, L: 80); CSF TB PCR positive |
No follow-up imaging Developed ATT drug hypersensitivity rash and steroid-induced hypertension |
| 6 | 12/F | Fever, neck pain, vomiting, seizures | VA: 6/6 both eyes; Pupils: Reactive to light; Fundus: Bilateral early papilledema. |
- Leptomeningeal enhancement in suprasellar, ambient, Sylvian cisterns, optic chiasma, brainstem, cisternal segments of V, VII, VIII cranial nerves - RELs in bilateral cerebellar hemispheres - Hydrocephalus - TB left hip |
Protein 30 mg/dL; Glucose 51 mg/dL |
VP shunt done for hydrocephalus No follow-up imaging |
EOM: Extraocular movement, VA: Visual acuity, CSF: Cerebrospinal fluid, MRI: Magnetic resonance imaging, REL: Ring-enhancing lesion, WBC: White blood cell, N: Neutrophil, L: Lymphocyte, ATT: Antitubercular therapy, VP: Ventriculoperitoneal, CNS: Central nervous system, TB: Tuberculosis, PCR: Polymerase chain reaction, F: Female.
All six patients were females and the median age involved was 15 years (range: 3–36 years). Three of the six patients (50%) were previously diagnosed with TBM and were already on antituberculous treatment (ATT) at the time of onset of visual symptoms. The mean time interval between the initiation of ATT and the development of visual symptoms was 5.67 months (±2.05 months). One patient had co-infections with retroviral and hepatitis-B infections.
The severity of TBM using the Medical Research Council staging system[3] is as below - stage I (patient fully conscious with no deficits) – 3 patients; stage II (minor neurological deficits with little to no impairment in sensorium) – 2 patients and; stage III (severe sensorium impairment ± convulsions/deficits) - one. Three of six patients presented with seizures including the one with immunocompromised status. The severity of vision impairment was categorized using the World Health Organization definition.[4] At presentation, 4/6 patients (66.67%) had visual impairment or blindness (one mild, two moderate, and three complete vision loss). One of the six patients had right third nerve palsy and another had bilateral sixth nerve palsy.
The patients exhibited ring-enhancing lesions (RELs), primarily conglomerate or coalescent, in the suprasellar cistern, with or without accompanying leptomeningeal enhancement in the chiasmal area [Figures 1-6]. Other findings included RELs in various supra- or infratentorial regions, vasculitic infarcts, and hydrocephalus.
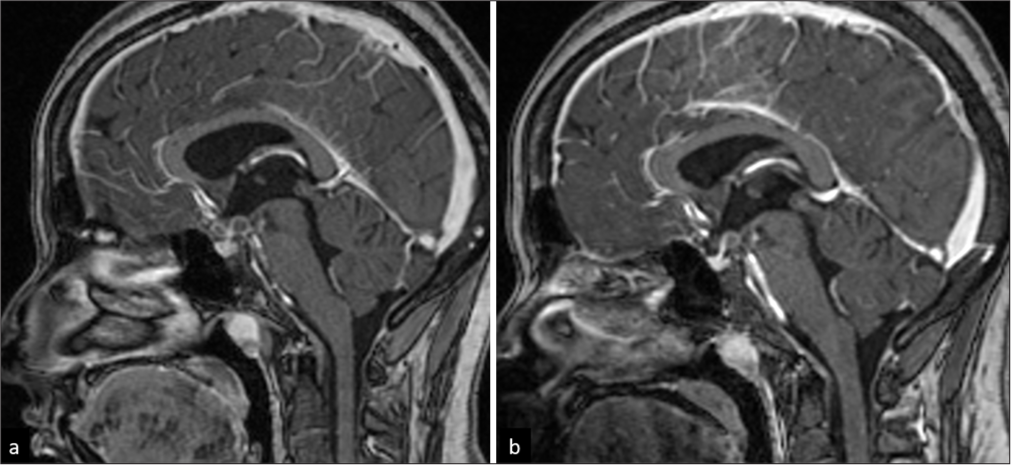
- A 20-year-old female with optochiasmatic tuberculomas. (a) Sagittal T1-weighted post-contrast image shows few ring-enhancing lesions in the chiasmatic cistern. (b) Mild reduction in size of the lesions on 3-year follow-up.
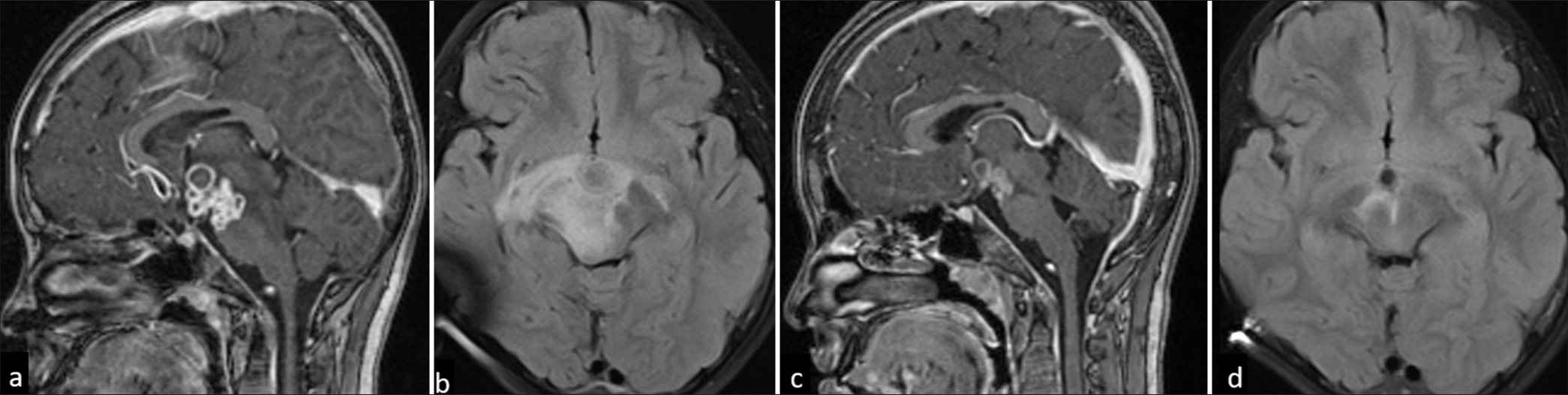
- An 11-year-old female with optochiasmatic tuberculomas. (a) Sagittal T1-weighted post-contrast image shows multiple conglomerate ring-enhancing lesions in hypothalamus, inferior third ventricle, suprasellar and interpeduncular cisterns. (b) Fluid-attenuated inversion recovery (FLAIR) axial image showing edema along the bilateral optic tracts and mid-brain. A tuberculoma with partial FLAIR suppression is seen in the interpeduncular cistern. (c and d) Reduction in size and number of lesions and edema on 1-year 3-month follow-up.

- A 20-year-old female with tuberculosis meningitis with optochiasmatic involvement. (a) Axial T1-weighted post-contrast image shows coalescent ring-enhancing lesion in the left anterolateral recess of suprasellar cistern. (b) Heavily T2-weighted SPACE axial sequence shows the lesion compressing the optic chiasma on the left side (arrow). (c) Reduction in size on 1 year 4 months follow-up and (d) Small residual lesion on imaging after 8 years.
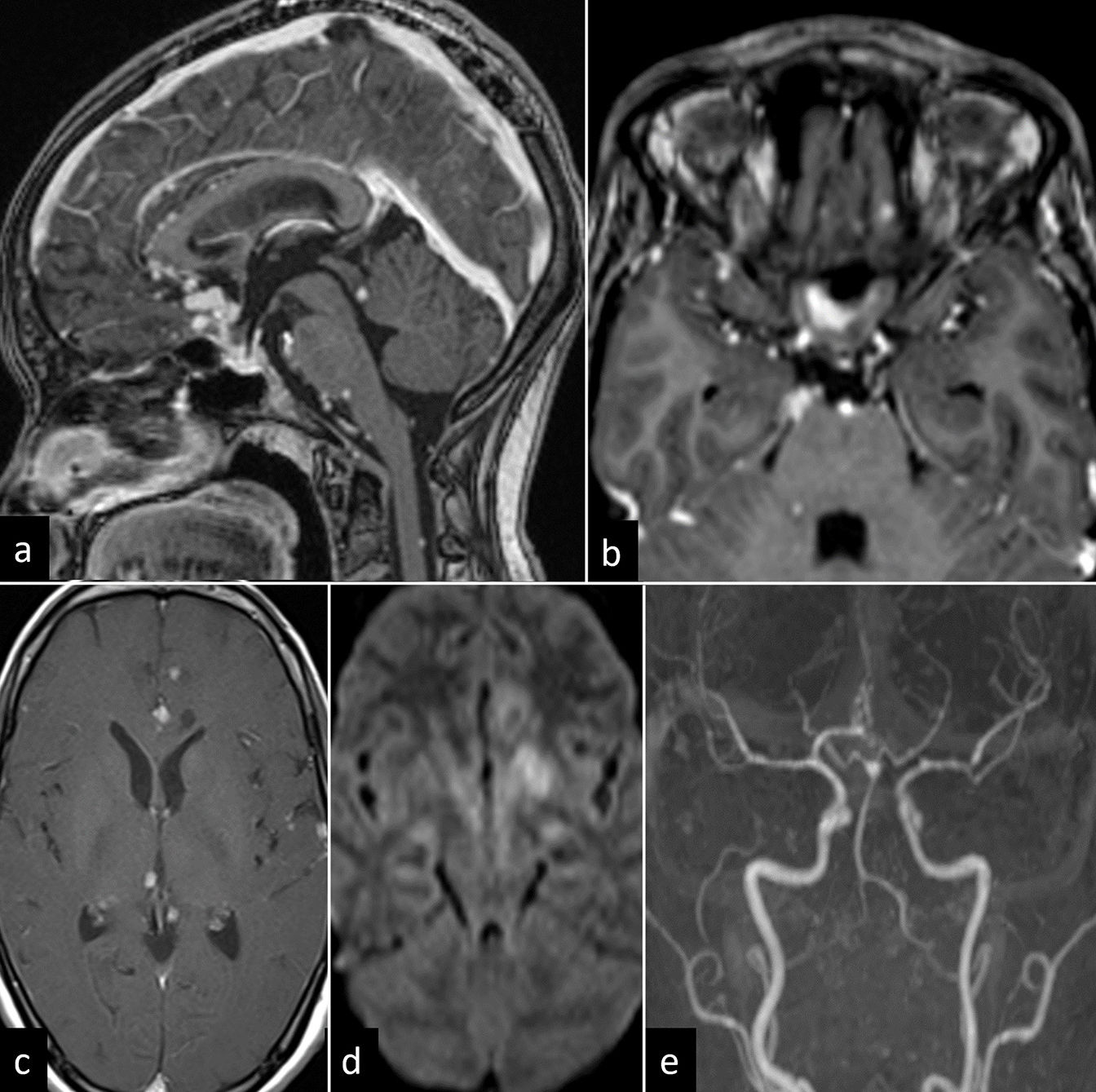
- A 36-year-old female with tuberculosis meningitis and vasculitis. (a) Sagittal T1-weighted post-contrast image shows leptomeningeal thickening with conglomerate ring-enhancing lesions in anterior commissure, optic chiasm, and infundibulum. Few nodular enhancing lesions are noted in midbrain tectum at the level of inferior colliculus, pons, and posterior surface of cervical cord. (b and c) Axial T1-weighted post-contrast image shows thick exudates along optic chiasma and multiple ring-enhancing lesions in anterior interhemispheric fissure, bilateral cerebral hemispheres, and right thalamus. (d) Diffusion-weighted image shows acute infarct in the left basal ganglia. (e) Time-of-flight angiography shows luminal narrowing in bilateral middle cerebral, anterior cerebral, and posterior cerebral arteries (left more than right).
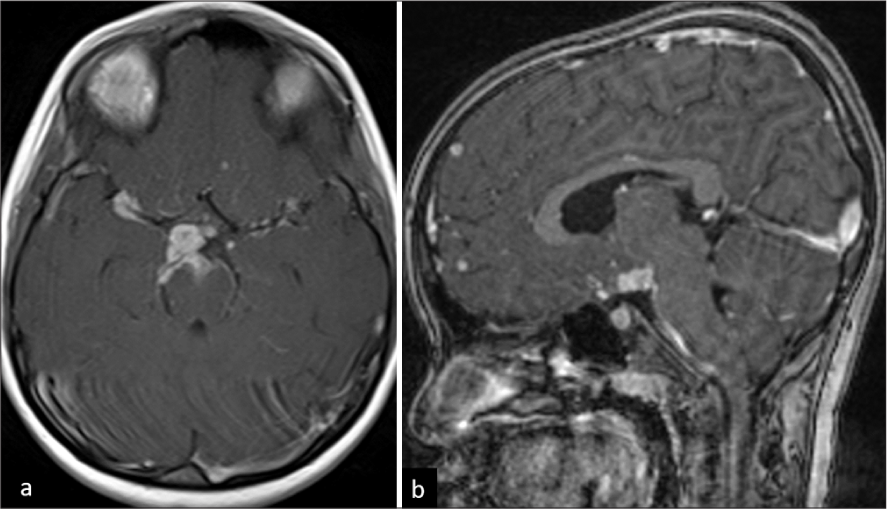
- A 6-year-old female child with tuberculosis meningitis. (a) Axial T1-weighted post-contrast image shows thick basal exudates in bilateral Sylvian cisterns (right > left), suprasellar and interpeduncular cisterns. (b) Sagittal T1-weighted post-contrast image shows conglomerate ring-enhancing lesions (RELs) in supra-optic recess and hypothalamic regions. Few RELs are noted in the frontal lobe.
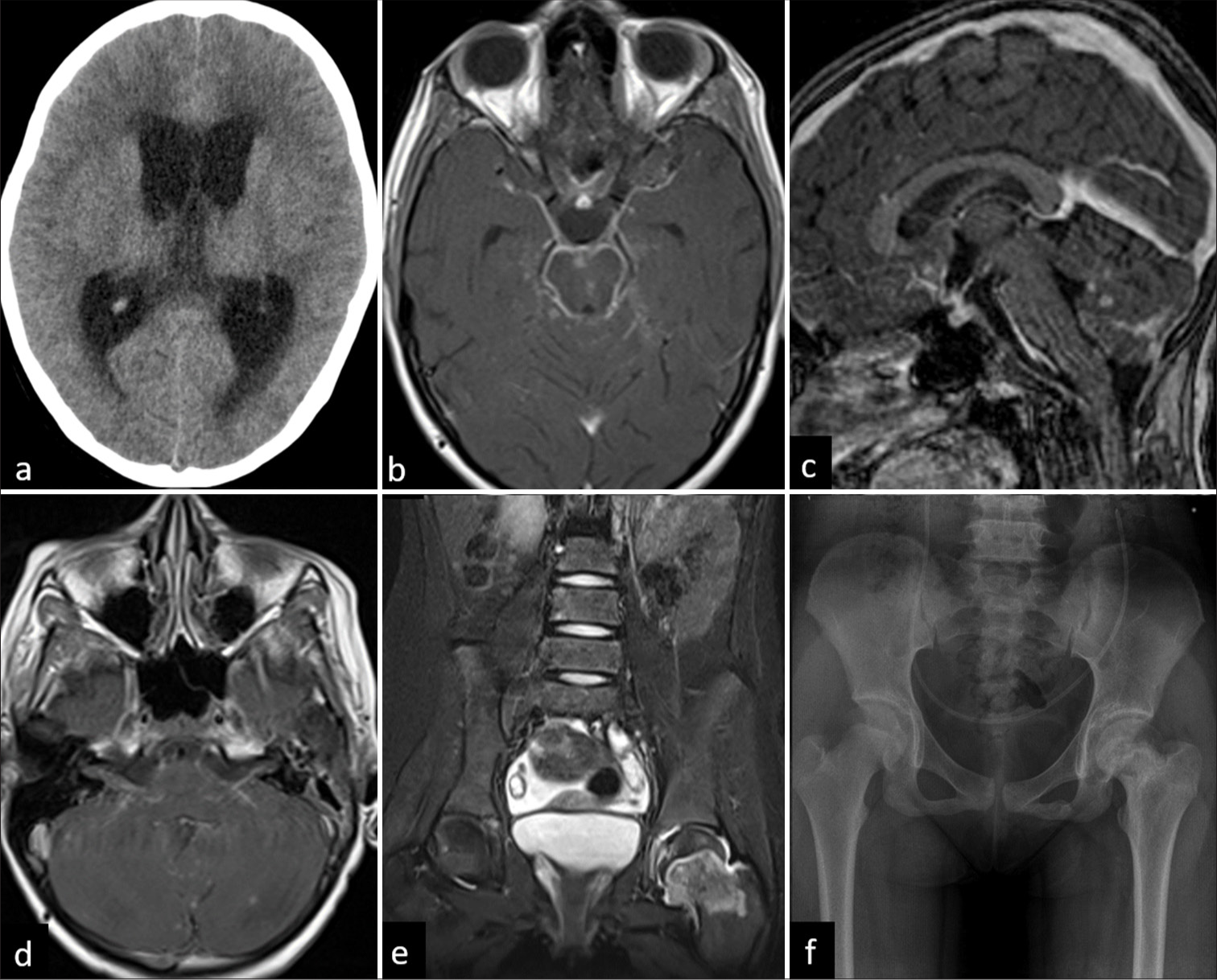
- A 12-year-old female child with optochiasmatic arachnoiditis. (a) Axial unenhanced computed tomography showing hydrocephalus. (b) Axial T1-weighted post-contrast image shows leptomeningeal enhancement in basal cisterns and chiasmatic region. (c) Sagittal T1-weighted post-contrast image shows ring-enhancing lesions in bilateral cerebellar hemispheres. (d) Axial T1-weighted post-contrast image shows enhancement along cisternal portion of bilateral seventh and eighth nerve complex. The same patient had tuberculosis left hip joint. (e) Coronal T2-fat suppressed image of pelvis shows marrow edema in the left femoral neck with mild joint effusion. (f) X-ray pelvis on 1-year follow-up showing progression of the disease with sclerosis of subchondral region and neck of femur.
All patients received ATT and steroids (dexamethasone or prednisolone for 4 weeks, tapered thereafter). Symptomatic treatment, such as antiepileptics for those with seizures, was also administered. Four out of six patients (66.67%) had associated hydrocephalus and underwent ventriculoperitoneal shunting. One patient had previously undergone surgical excision of a cerebellar tuberculoma. Three of the six patients underwent follow-up imaging, which showed a significant reduction in the size and number of the tuberculomas and a reduction in edema.
DISCUSSION
OCA was first described as a clinical entity in 1929 and was known as the “Syndrome de Balado.”[1] The first reports were in patients with Meningovascular syphilis followed by the first documented case of tuberculous OCA reported by Yuhl and Rand in 1951.[5] Other reported etiologies of OCA include rheumatoid pachymeningitis, sarcoidosis, and Epstein–Barr viral infection.[6]
The optochiasmatic region predominantly consists of a suprasellar cistern and its contents. The suprasellar cistern is the subarachnoid space around the optic chiasm above the sella turcica and consists of the chiasmatic cistern, the interpeduncular cistern, and the cistern of lamina terminalis. It is a vital CSF space that contains the circle of Willis, optic nerves, chiasm, hypothalamus, and pituitary stalk.[7]
Tuberculous meningitis has a strong predilection for basal parts of the brain. Exudates of tuberculous meningitis present in the basal cisterns (interpeduncular, suprasellar chiasmatic, and Sylvian cisterns) result in OCA and/or tuberculomas.[8] It may result from the infectious process itself but is more usually seen as a paradoxical reaction to ATT.[9,10] There is a thickening of the arachnoid mater at the base of the brain with thick exudates surrounding the optic chiasm and the adjacent cranial nerve roots. Apart from the basal exudates, the edema, vasculitis, infarction, and expansion of tuberculomas may also compress and occlude the blood supply to the optic nerve and the optic chiasma leading to severe vision loss.[2,11] However, other potential causes of visual impairment in patients with tuberculosis should also be ruled out which include primary optic neuritis, toxic optic neuropathy (often associated with ATT drug ethambutol), chiasmal compression secondary to hydrocephalus and increased intracranial pressure, and chiasmal prolapse.[12]
This clinical entity more frequently affects young adults. Previous studies have identified that female sex, younger age, and raised CSF protein content as predictors for developing OCA.[1,6] In our study, all six patients were females, and the median age involved was 15 years (range 3–36).
Frequently, optochiasmatic tuberculoma and OCA develop paradoxically while a patient is being treated with anti-TB drugs.[13,14] Visual loss during the treatment of TBM may be related to either drug-related toxicity or arachnoiditis or tuberculoma involving the anterior optic pathway.[15,16] The mean time interval between the initiation of ATT and the development of visual symptoms was 5.67 months (±2.05 months) in our case series.
These findings are similar to that of a large series by Aaron et al.,[6] who analyzed 163 patients with TBM admitted over 6 years and found 23 (14%) developing optic chiasmal atrophy. Of these 23 patients, 18 (78%) developed OCA while on ATT, and 5 (22%) were newly diagnosed with TBM. Among those already receiving treatment, 12 (52%) were on ATT alone, while 6 (26%) had received a combination of steroids and ATT at varying doses and duration. The average time from TBM diagnosis to the onset of visual symptoms was 6.4 months. They identified female sex, age under 27 years, and protein content in the CSF >260% mg as significant risk factors for developing this complication. At 6-month follow-up, among those treated with steroids and ATT, 17% showed improvement, and no further deterioration in visual acuity was noted in 52% of patients. In another series by Sinha et al.,[17] the meantime for developing paradoxical optochiasmatic tuberculomas was even shorter, with the onset occurring 41 days after initiating anti-TB therapy in 8 patients studied. Of the eight patients, paradoxical optochiasmatic tuberculoma led to vision deterioration in all, with 6 experiencing severe vision loss (visual acuity of 6/60). All patients received an extended course of dexamethasone for 6 weeks in addition to ATT. On follow-up, two patients died at 2 and 7 months and follow-up imaging in the remaining patients showed resolution of the optochiasmatic tuberculoma. At 9 months followup, three patients had complete vision recovery, while three others had partial improvement.
Visual impairment is the most devastating complication because vision loss persists, unlike the other cranial nerve involvement, which might improve or recover completely with time. The prognosis is poor if there is a delay in the diagnosis and/or treatment. Treatment in itself is challenging and involves anti-TB drugs and a prolonged course of steroids (often >8 weeks). In the central nervous system, TB with visual impairment, ethambutol is often replaced with ofloxacin, levofloxacin, or streptomycin as a first-line drug to prevent further visual deterioration.[9] Corticosteroids are the primary treatment for paradoxical reactions, though other immunomodulators such as thalidomide and infliximab are being investigated as potential alternatives.[1,12] In cases with non-response or failure of medical management, surgical exploration of the optochiasmatic region and decompression/release of the optic chiasma/tracts from the surrounding leptomeningeal fibrosis has been described to be beneficial as it relieves the mechanical factor or at least arrest the optic atrophy.[7,13]
CONCLUSION
The development of visual impairment in a patient receiving ATT should raise concerns for optochiasmatic involvement apart from drug toxicity. The prompt management requires a CE MRI of the brain and swift initiation of steroid treatment that significantly improves the prognosis, as evidenced by the reduction in lesion size and neurological function in most of our cases. However, challenges remain, especially in regions with a high burden of TB, where diagnosis may be delayed due to the non-specific nature of the symptoms. Timely intervention can reduce the long-term impact on vision and avoid potentially treatable vision loss and/or neurological deficits.
Ethical approval:
The research/study was approved by the Institutional Review Board at JIPMER IEC, number JIP/IEC/2021/092, dated September 13, 2021.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Tuberculous optochiasmatic arachnoiditis: A devastating form of tuberculous meningitis. Expert Rev Anti Infect Ther. 2011;9:719-29.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxical reaction in tubercular meningitis resulting in involvement of optic radiation. Indian J Ophthalmol. 2009;57:139-41.
- [CrossRef] [PubMed] [Google Scholar]
- International Statistical Classification of diseases and related health problems, 11th revision, Chapter 9 D90: Visual impairment including blindness. World Health Organization. Available from: https://www.who.int/classifications/icd/en [Last accessed on 2023 Jan 09]
- [Google Scholar]
- Tuberculous opticochiasmatic arachnoiditis; report of a case. J Neurosurg. 1951;8:441-3.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculous optochiasmatic arachnoiditis. Neurol India. 2010;58:732-5.
- [CrossRef] [PubMed] [Google Scholar]
- Opto-chiasmatic arachnoiditis; surgical treatment and results. J Neurosurg. 1951;8:355-9.
- [CrossRef] [PubMed] [Google Scholar]
- Optochiasmatic tuberculoma as the sole manifestation of late recurrent tuberculosis. Rev Inst Med Trop Sao Paulo. 2012;54:229-30.
- [CrossRef] [PubMed] [Google Scholar]
- Neuro-ophthalmic manifestations of tuberculosis. Eye (Lond). 2022;36:15-28.
- [CrossRef] [PubMed] [Google Scholar]
- Optochiasmatic tuberculomas: A vision-threatening paradoxical response in tuberculous meningitis. Turk Neurosurg. 2012;22:246-9.
- [Google Scholar]
- Optic pathways tuberculoma mimicking glioma: Case report. Surg Neurol. 2003;60:349-53.
- [CrossRef] [PubMed] [Google Scholar]
- The neuro-ophthalmology of tuberculosis. Neuroophthalmology. 2023;48:73-92.
- [CrossRef] [PubMed] [Google Scholar]
- Optochiasmatic tuberculomas as a paradoxical reaction to treatment for meningeal tuberculosis. Rev Neurol. 2018;66:286-8.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxically developed optochiasmatic tuberculoma and tuberculous lymphadenitis: A case report with 18-month follow up by MRI. South Med J. 2006;99:388-92.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxical growth of optochiasmatic tuberculoma during the treatment of tuberculous meningitis. Curr Health Sci J. 2014;40:225-7.
- [Google Scholar]
- Tuberculous optochiasmatic arachnoiditis and vision loss. Neurology. 2016;87:1845.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxical vision loss associated with optochiasmatic tuberculoma in tuberculous meningitis: A report of 8 patients. J Infect. 2010;60:458-66.
- [CrossRef] [PubMed] [Google Scholar]






