Translate this page into:
Neutrophil-lymphocyte ratio as a predictor of outcome following traumatic brain injury: Systematic review and meta-analysis
*Corresponding author: Dr. Amit Agrawal, Department of Neurosurgery, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India. dramitagrawal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mishra RK, Galwankar S, Gerber J, Jain A, Yunus M, Cincu R, et al. Neutrophil-lymphocyte ratio as a predictor of outcome following traumatic brain injury: Systematic review and meta-analysis. J Neurosci Rural Pract 2022;13:618-35.
Abstract
Objectives:
The neutrophil-to-lymphocyte ratio (NLR) is a simple and routinely performed hematological parameter; however, studies on NLR as a prognostic tool in traumatic brain injury (TBI) have yielded contradictory results.
Materials and Methods:
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items in the Systematic Review and Meta-Analysis guidelines 2020. Electronic databases of PubMed, Cochrane Library, Web of Science, and Scopus were searched. The population consisted of TBI patients in the absence of moderate and severe extracranial injury. Day 1 NLR was taken for the analysis. The outcomes evaluated were mortality and the Glasgow Outcome Scale (GOS). No restrictions were placed on the language, year and country of publication, and duration of follow-up. Animal studies were excluded from the study. Studies, where inadequate data were reported for the outcomes, were included in the qualitative synthesis but excluded from the quantitative synthesis. Study quality was evaluated using the Newcastle-Ottawa scale (NOS). The risk of bias was estimated using the Cochrane RoBANS risk of bias tool.
Results:
We retrieved 7213 citations using the search strategy and 2097 citations were excluded based on the screening of the title and abstract. Full text was retrieved for 40 articles and subjected to the eligibility criteria, of which 28 were excluded from the study. Twelve studies were eligible for the synthesis of the systematic review while seven studies qualified for the meta-analysis. The median score of the articles was 8/9 as per NOS. The risk of selection bias was low in all the studies while the risk of detection bias was high in all except one study. Ten studies were conducted on adult patients, while two studies reported pediatric TBI. A meta-analysis for GOS showed that high NLR predicted unfavorable outcomes at ≥6 months with a mean difference of −5.18 (95% confidence interval: −10.04, −0.32); P = 0.04; heterogeneity (I2), being 98%. The effect estimates for NLR and mortality were a mean difference of −3.22 (95% confidence interval: −7.12, 0.68), P = 0.11, and an I2 of 85%. Meta-analysis for Area under the curve (AUC) receiver operating characteristic of the included studies showed good predictive power of NLR in predicting outcomes following TBI with AUC 0.706 (95% CI: 0.582–0.829).
Conclusion:
A higher admission NLR predicts an increased mortality risk and unfavorable outcomes following TBI. However, future research will likely address the existing gaps.
Keywords
Neutrophil-lymphocyte ratio
Traumatic brain injury
Outcome
Systematic review
Meta-analysis
INTRODUCTION
Traumatic brain injury (TBI) is a considerable noncommunicable disease and has emerged as a silent epidemic that affects economically and socially productive individuals. TBI is a complex and dynamic entity with its effects days after the injury. The primary injury primarily determines the outcome of the TBI patient at the time of impact, marked by brain damage, loss of function, and death. Apart from high mortality, there are significant complications in the individuals who survive, including poor functional outcomes, dementia, and infections.[1-3] Jennett and Bond created the Glasgow Outcome Scale (GOS) as a 5-point objective measurement tool in 1975 to assess the TBI outcome.[4] The goal of successful management in TBI is to prevent secondary injury. The key factors determining the outcome of TBI are age, gender, and immediate impact; however, these are non-modifiable.[2] Immune changes in post-TBI are potentially modifiable factors and provide a therapeutic window to limit secondary brain injury and improve the outcome following TBI.[1,5] Several prognostic indicators and tools such as IMPACT and CRASH are developed to guide the management and predict the outcome in TBI patients. These tools are used to predict short-term and long-term mortality and functional outcome. However, it is always not easy to obtain all the elements in the prognostic tools in different hospital settings. Accordingly, researchers attempted to identify simple biomarkers as hematological parameters to predict the outcomes.
Studies in trauma immunology and animal studies suggest the potential role of neutrophils in adverse sequelae following TBI.[5] Neutrophils are critical components of the innate immune system which is the first defense against microbial infection. TBI is characterized by the increased immune response following injury and later by immune depression, leading to respiratory failure, multiorgan dysfunction, and nosocomial infection.[6,7] Evidence from immunology studies suggests that neutrophils play a linking role between the innate immune response and chronic immune response.[6] Several studies have attempted to explore the utility of simple hematological investigations in predicting the outcomes following TBI. Some studies have shown that neutrophillymphocyte ratio (NLR) has predictive power similar to GCS in predicting mortality and GOS outcome.[8-10] However, some studies suggest that the predictive performance of NLR is not superior to other predictive biomarkers.[11,12] The present study aims to critically assess the available evidence and identify the knowledge gaps about NLR in predicting TBI outcomes.
MATERIALS AND METHODS
We have conducted the present study as per the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement published in 2009 and updated in 2020.[13]
Study design
This is a prognostic systematic review to evaluate the available evidence on NLR as a predictor of outcomes following TBI.
Eligibility criteria
Published studies were identified through electronic searches to answer the review question–“What is the prognostic value of the NLR as a predictor of outcome following a TBI?” Studies were screened for eligibility according to the PICO question framework.
Population (P): Patients with TBI in whom NLR was measured at admission and/or at serial intervals
Intervention (I): Nil
(C): Nil
Outcome (O):
Primary: Mortality and GOS
Secondary outcomes included length of hospital stay, ventilatory days, and long-term functional outcomes
The GOS was dichotomized according to the standard classification into favorable outcome (GOS I-III) and unfavorable outcome (GOS IV-V). GOS is a 5-point scale described by Jennett and Bond in 1975.[4]
Eligible study designs included a prospective, cohort, retrospective, and observational study and a case series. We applied no restrictions on the minimum follow-up duration reported and study settings. There was no restriction based on the year of publication and language to minimize the risk of publication bias. Only published studies were eligible for inclusion and we sought only published data. Unpublished studies, review articles, animal studies, letters to editors, and conference abstracts were not included in the study. The study screening and selection were made as per Cochrane Collaboration Methodology.[14] The studies where NLR was not reported were excluded from the study. The studies where the outcome of interest was not studied or not reported were excluded from the study. Studies based on TBI type and severity were included in the study. Studies reporting patients with extracranial injuries of abbreviated injury score >3 severity were excluded from the review. The second publication from the same study was also excluded from the study. There was no restriction on the age group to include a study in the systematic review.
Information sources
Relevant articles were identified by searching the electronic databases PubMed, Cochrane Library, Web of Science, and Scopus. RM and AJ performed the search, and the differences were resolved by discussion with a third reviewer (AA). In addition to the electronic search, the studies cited in the included studies, institutional repositories, relevant neurosurgery journals, and manual search using the Google Scholar website related to the subject searched. The searches were first conducted on September 11, 2021, and updated on October 20, 2021. Only human studies were selected from the electronic database wherever such a filter was available. All results were screened in other databases where such a filter option was not available. There was no restriction applied to the language of publication or publication date. We screened the reference list of the relevant articles and systematic reviews on similar topics to recognize additional eligible articles. After removing the duplicates, full-length articles were retrieved and assessed for qualitative and quantitative synthesis eligibility.
Search strategy
Line-by-line search strategy for all the databases is presented in Supplementary File S1. The search strings were validated as shown in the PUBMED search strategy. As the search was more sensitive but less specific with the use of MeSH and keywords for NLR and TBI, keywords for the search of outcome measures were not used in the search strategy. The search strategy for PUBMED is as follows: ((“Neutrophils”[MeSH Terms] OR “Leukocytes”[MeSH Terms] OR “Lymphocytes”[MeSH Terms] OR “Leukocytosis”[MeSH Terms] OR “Lymphocytosis”[MeSH Terms] OR “neutrophil*”[Text Word] OR “leukocyte*”[Text Word] OR “TLC”[Text Word] OR “total leukocyte count*”[Text Word] OR “lymphocyte*”[Text Word] OR “lymphocyte count*”[Text Word] OR ((“neutrophil leukocyte*”[Text Word] OR “neutrophil lymphocyte*”[Text Word]) AND (“ratio”[All Fields] OR “ratios”[All Fields] OR “ratios”[All Fields] OR “ratios”[All Fields]))) AND (“brain injuries, traumatic”[MeSH Terms] OR “Brain Concussion”[MeSH Terms] OR “TBI*”[Text Word] OR “head injury*”[Text Word] OR “brain injury*”[Text Word] OR “contusion*”[Text Word] OR “cerebral injury*”[Text Word] OR “cortical injury*”[Text Word] OR “Hematoma”[Text Word])) AND (humans[Filter]).
Selection process
Two reviewers (RM and AA) independently screened the title and abstract of each record for eligibility. The discrepancy was first resolved with mutual discussion and then with the consensus of the third reviewer (AJ).
Data extraction and effect estimates
Two reviewers (RM and AJ) performed independent data extraction using the piloted data abstraction form guided by Cochrane recommendations.[14] In the event of a discrepancy, the third reviewer (AA) resolved the conflict unanimously. The data collected from the studies included study details, study design, sample size, country and journal of publication, study objectives, statistical measures, inclusion and exclusion criteria, outcome measures, follow-up, subgroups analyzed, results, and critical conclusions. The effect estimates reported in the studies as mean and standard deviation were used for quantitative synthesis. In studies where the median was reported with a large sample size, we calculated the mean and standard deviation according to McGrath et al. and Cochrane handbook.[15,16] Area under the curve (AUC) reported in the studies was used to compute AUC meta-analyses of effect estimates. GOS outcome was considered most important for interpreting the review’s conclusions as it was most objectively reported and not affected by other coexisting conditions. Details were collected on the setting of the study and participant characteristics, whether adult or pediatric, isolated TBI or polytrauma, and severity of TBI. GOS outcome was dichotomized as favorable (GOS I-III) and unfavorable (GOS IV-V). Effect estimates for NLR were reported as the mean difference with 95% CI.
Study quality and risk of bias assessment
Two authors (RM and AA) evaluated the study quality using the Newcastle-Ottawa scale (NOS)[17] and the risk of bias using the RoBANS[18] risk of bias tool for non-randomized studies. Any conflicts in the assessment were mostly resolved with mutual consensus and in some cases with the involvement of the third author (AA). Newcastle-Ottawa quality assessment scale[17] is used to evaluate the study quality based on three domains of selection, comparability, and outcomes and consists of a set of eight questions. The question to assess comparability can have 2 points while the rest of the questionnaire items can have a maximum of 1 point each. The maximum score for a study in NOS is nine. For the present review, we considered a study with a score ≥ 6 as good quality and consistent. RoBANS[18] risk of bias tool is a 6-item tool to evaluate the risk of bias in selection, confounding, attrition, performance, and reporting bias domains in a non-randomized study.
Synthesis methods
Quantitative synthesis was done from the study’s published data and effect estimates were reported for the outcome measures specified wherever available. Studies in which data were not reported or could not be computed from the reported data in a dichotomized manner were included in the systematic review but were not suitable for the quantitative synthesis. In studies where an extended Glasgow Outcome Score was reported as the outcome measure, GOS was computed. Studies that have reported outcome tools other than GOS and Extended GOS were included in the systematic review but excluded from the quantitative synthesis. The systematic review is presented as a narrative synthesis. We used the random-effects model to compute the effect estimates for the GOS and mortality. NLR was the continuous variable and the inverse variance statistical method was used. I2 statistics described the heterogeneity in the studies, where low heterogeneity meant an I2 < 40%. P < 0.05 was considered statistically significant. Funnel plots were studied to identify the publication bias and variability in the studies. We did sensitivity analysis as a subgroup analysis to explore the reasons for heterogeneity.
Ethics and data
This study did not involve any human participants and did not require ethical approval. The systematic review was prospectively registered with PROSPERO Id CRD42022285439.
RESULTS
Study selection
Seven thousand two hundred and thirteen citations were obtained from the electronic database using the search strategy. Two thousand one hundred and thirty-six records were screened after removing duplicates. After screening the title and abstract, 2097 citations were excluded from the study. The full text of 40 articles was retrieved and assessed for eligibility, of which 28 were excluded from the study. The list of excluded studies with the reason is presented in [Table 1]. Twelve articles were eligible for inclusion in the systematic review.[8-11,19-26] Eight articles were eligible for quantitative synthesis.[8-11,19,20,22,24] The reason for articles excluded from meta-analysis is presented in [Table 2]. The study screening and selection process are shown as flow diagram in [Figure 1].
| Study Id/Year/Country | Reason for exclusion |
|---|---|
| Keskil et al./1994/Turkey[39] | Study explored leukocytosis in TBI and did not report separately the neutrophils, lymphocytes, or NLR. |
| Holmin et al./1998/Sweden[40] | Study explored the inflammation in contused brain tissue and did not match the eligibility criteria for the present SR |
| Rovlias and Kotsou/2001/Greece[41] | Assessed WBC count in severe head injury but did not report neutrophils and lymphocytes as outcome in TBI |
| Pagowska-Klimek et al./2007/Poland[42] | Assessed post-injury effects on neutrophils and lymphocytes and not the outcome. Did not match the eligibility criteria of present SR |
| Gürkanlar et al./2009/Turkey[33] | Assessed WBC count in severe head injury but did not report neutrophils and lymphocytes as outcome in TBI |
| Fitrolaki et al./2013/Greece[43] | Assessed CD 64 expression of neutrophils and sepsis in TBI. Did not match the eligibility criteria of present SR. |
| Liao et al./2013/China[44] | Assessed oxidative burst of neutrophils and did not report the NLR and outcome. |
| Wang et al./2014/Taiwan[45] | Assessed neutrophils apoptosis as predictive outcome and not NLR. |
| Gusdon et al./2017/USA[46] | Assessed role of leukocytes in perihematomal growth |
| Liu et al./2018/China[47] | Review article |
| Lattanzi et al./2019/Italy[48] | Systematic review on stroke and neutrophils |
| Needham et al./2019/United Kingdom[49] | Review article |
| Von Leden et al./2019/USA[50] | Review article |
| Wang et al./2019/China[51] | Assessed NLR as predictor of hematoma growth and not the outcomes required for the present SR |
| Yu et al./2019/China[52] | Systematic review on leukocytosis in intracerebral hemorrhage and did not assess TBI |
| Alexiou et al./2020/Greece[53] | Assessed NLR to predict the CT scan in TBI and did not match eligibility criteria of present SR |
| Bai et al./2020/China[54] | Assessed NLR in stroke and not in TBI |
| Chen et al./2020/China[55] | Assessed post-operative NLR after hematoma evacuation. The study was not on TBI and admission NLR was not assessed to predict the outcome. |
| Kaur et al./2020/India[56] | Systematic review on phytotherapeutic intervention in neuroinflammation |
| Korobey et al./2020/USA[12] | Symposium paper |
| Kusuma et al./2020/Indonesia[57] | The study evaluated NLR with CRP and ESR in TBI. There were no outcomes assessed. |
| Li et al./2020/China[58] | Assessed NLR and DWI and did not match eligibility criteria of present SR |
| Sabouri et al./2020/Iran[59] | Review article |
| Sadaka et al./2020/USA[60] | Symposium paper and duplicate |
| Zhang et al./2020/China[61] | Participants had chronic subdural hematoma |
| Gul et al./2021/Turkey[62] | Did not assess NLR |
| Menon et al./2021/India[63] | Assessed NLR in ICH and not in TBI |
| Radu et al./2021/Romania[64] | Assessed NLR in ICH and not in TBI |
TBI: Traumatic brain injury, NLR: Neutrophil-lymphocyte ratio, ICH: Intracerebral hemorrhage, DWI: Diffusion-weighted imaging, CT: Computed tomography, CRP: C-reactive protein, ESR: Erythrocyte sedimentation rate
| Study Id | Reason for exclusion |
|---|---|
| Dolmans et al., 2020[21] |
Data not presented in each arm of the groups compared |
| Kimball et al., 2020[26] |
Data on NLR were not reported for survivors versus non-survivors. Data on outcome dichotomized as favorable and non-favorable were not present. |
| Mukherjee et al., 2020[23] |
The study did not reported data among survivors versus non-survivors and outcome measure used was PCPCS and not GOSE-pediatric score. |
| Le Bail et al., 2021[25] |
Data on NLR and functional outcome were not reported |
NLR: Neutrophil-lymphocyte ratio, GOSE: Extended Glasgow outcome scale, PCPCS: Pediatric cerebral performance category scale score

- PRISMA flow diagram showing study search, screening, and selection process.
Study characteristics
All included studies were published in English. Four studies were published from China and one each from India, Australia, the United States of America, the United Kingdom, the Netherlands, Poland, Korea, and France.[8-11,19-26] Only one study was a prospective and cohort study; the rest were retrospective studies.[11] All the studies had community-dwelling participants. Two studies reported pediatric TBI patients.[23,26] Rest studies had adult participants.[8-11,19-22,24,25] Participants in 10 studies had isolated TBI.[8-11,19,21,23-26] In contrast, two studies reported on patients with TBI and extracranial injuries.[20,22] Except for two studies, all studies reported severe TBI patients (GCS ≤8).[20,25] All studies reported day 1 NLR measured at admission, and three studies also mentioned serial measurements of NLR.[8,9,26] Mean follow-up in the included studies was 7.5 months (range: 5 days–18 months). The total number of patients evaluated in the qualitative review was 3975. The study characteristics are presented in tabular form in [Table 3]. Critical analysis of the included studies and results of individual studies with effect estimates is presented in [Table 4]. [Table 5] shows the critical variables in the included studies and literature matrix depicting the gap in knowledge in the existing literature.
| Study Id | Country | Study type | Aim of study | Sample size | Study duration | Population description and setting | Inclusion criteria | Exclusion criteria | Defined management and NLR measurement | Time of NLR measurement | Tools for outcome measurement | Outcomes measured | Subgroups reported | Follow-up duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al., 2018[24] |
China | Retrospective observational study | To assess the value of NLR to predict outcomes in TBI | n=688 | January 2007–April 2012 | Adult with isolated TBI patients attending single institution | 1. Isolated TBI; 2. GCS≤8; 3. Time from injury to admission≤6 h | 1. Age<18 years; 2. Time from injury to admission>6 h; 3. Prior head trauma; 4. Neurological disease including stroke; 5. Antiplatelets, anticoagulants, steroids, immunosuppressant; 6. Systemic disease | Blinded assessment of CT images and lab data; authors mentioned protocol of management and CT classification using Traumatic Coma Data Bank criteria | At admission; neutrophil count divided by lymphocyte count | Medical charts and telephonic interviews | Functional outcome as GOS (Favorable-GOS I-III; Unfavorable-GOS IV-V); mortality | Age, temperature, GCS, neurological deterioration, seizure, mechanical ventilation, TBI severity and NLR on GOS, and survival at 1 year post-trauma | 1 year |
| Chen et al., 2019[9] |
China | Retrospective observational study | To understand relationship between peak NLR and clinical outcome in severe TBI patients | n=316 | January 2013–January 2017 | Adult with isolated TBI patients attending single institution | 1. Isolated TBI; 2. GCS≤8; 3. Time from injury to admission≤24 h; 4. Two NLR measurements done at≥3 days apart. | 1. Age<16 years; 2. Time from injury to admission>24 h; 3. Prior head trauma; 4. Neurological disease including stroke; 5. Systemic diseases like uremia, tumor. 6. Missing data or loss to follow-up. | Management was guided by Brain Trauma Foundation guidelines 2007 | At admission and again within 12 days; neutrophil count divided by lymphocyte count; peak NLR defined as maximum NLR in 12 days. | Not mentioned | Functional outcome as GOS (Favorable-GOS I-III; Unfavorable-GOS IV-V) | Age, GCS, blood glucose, surgery in the first 24 h, length of hospital stay, day 1, and peak NLR with GOS at 1 year post-trauma | 1 year |
| Corbett et al., 2019[10] |
Australia | Retrospective observational study | To compare the prognostic ability of hematologic parameters with the IMPACT prognostic model. | n=388 | 2004–2016 | Adults with severe TBI requiring decompressive craniectomy | Adults with severe TBI requiring decompressive craniectomy | Not reported | Not reported | At admission | Medical database of two hospitals | Functional outcome as GOS (Favorable-GOS I-III; Unfavorable-GOS IV-V) | Hemoglobin, INR, DIC, aPTT, fibrinogen, and total leukocyte count | 18 months |
| Siwicka-Gieroba et al., 2019[8] | Poland | Retrospective observational study | To analyze the effect of NLR on outcomes of severe TBI | n=144 | Not reported | Adult with isolated TBI patients attending single institution | Consecutive adult patients with isolated severe TBI admitted to intensive care unit. | 1. Age<18 years; 2. Pregnant women; 3. Patients with drug overdoses; 4. Patients with history of neoplastic, cardiac, hepatic, or renal disease. | Management was guided by Brain Trauma Foundation guidelines 2007 and ICU management defined with ICP measurement and surgery details | At admission and serially for 6 days in the ICU | Medical charts and telephonic interviews | Extended Glasgow Outcome Score at 7, 28 days, and 6 months | Correlation of outcome and variables with different TBI types and correlation of GOSE with serially measured NLR for the first 6 days | 6 months |
| Zhao et al., 2019[22] |
China | Retrospective observational study | To evaluate the prognostic value of NLR in predicting 6 months outcome after TBI. | n=1291 | December 2004–December 2017 | Adult with TBI patients attending single institution | 1. Confirmed TBI on CT scan; 2. Age≥14 years; 3. Admission within 6 h after injury | 1. Injury to other body parts with abbreviated injury score≥3; 2. Penetrating brain injury | Management was guided by Brain Trauma Foundation guidelines 2007 and management guidelines of severe traumatic brain injury 2017, neurosurgery. | At admission | Medical charts and telephonic interviews | Functional outcome as GOS (Favorable-GOS I-III; Unfavorable-GOS IV-V) | Age, GCS, coagulopathy, TBI type, and mechanism of injury | 6 months |
| Dolmans et al., 2020[21] |
Netherlands | Retrospective observational study | To estimate routine blood parameters in severe TBI and its correlation with the outcome | n=255 | 2005–2015 | Adult with severe TBI admitted in two academic institutes | Severe TBI patients with GCS≤8 | <18 years | Not reported | At admission | Medical database of two hospitals | Functional outcome as GOS (Favorable-GOS I-III; Unfavorable-GOS IV-V); mortality at 30 days; length of hospital stay 30 days or less. | Individual parameters of laboratory variables, ISS | 3 months |
| Kim et al., 2020[20] |
Korea | Retrospective observational study | To assess the value of NLR to predict 1 year mortality after surgery for EDH and SDH | n=200 | September 2010–December 2018 | Adult TBI patients undergoing surgery for EDH and SDH in a single center | 1. Age>18 years; 2. Admission within 6 h of injury; 3. Surgery within 24 h of injury | 1. Reoperation; 2. Age<18 years; 3. Burr hole instead of craniotomy; 4. No surgery within 24 h of injury; 5. Conservative management. | Not reported | At admission | Medical database | Mortality at 1 year | Age, GCS, WBC, aPPT, INR, anesthesia time, surgery time, and reoperation within 24 h | 1 year |
| Kimball et al., 2020[26] |
USA | Retrospective observational study | To assess the value of NLR to predict outcome after TBI in pediatric patients | n=188 | January 2007–December 2017 | Pediatric TBI patients attending single institution | 1. Age 0–18 years; 2. Isolated TBI; 3. Blood investigation within 84 h after injury | 1. Severe clinically diagnosed comorbidities; 2. Prior neurological disease; 3. Anticoagulants, steroids, or immunosuppressants; 4. Systemic disease | Management as per guidelines | <12 h, 24 h, 48 h, and 72 h | Medical database | GOSE pediatric score | PTA, LOC | 86 days |
| Mukherjee et al., 2020[23] |
United Kingdom | Retrospective observational study | To assess the prognostic value of initial leukocytosis in predicting outcomes after isolated TBI in pediatric patients. | n=201 | June 2006–June 2018 | Pediatric isolated TBI patients attending single institution | 1. Age 0–16 years; 2. Blood investigation within 2 h of injury | Age>16 years, delayed investigation, patients without isolated TBI | Not reported | At admission within 2 h of injury | Medical database | Length of hospital stay and 6 months Pediatric Cerebral Performance Category Scale score | GCS, WBC, DLC, medical and surgical management, and CT brain | 6 months |
| Bilgi et al., 2021[11] |
India | Prospective observational study | To assess hematological parameters with 6 months GOSE outcome in TBI | n=96 | June 2019–November 2019 | Adult with isolated TBI patients attending single institution | 1. Age 18–60 years; 2. GCS≤12; 3. Isolated TBI | 1. Severe extracranial injuries; 2. Infection; 3. History of stroke, autoimmune disease; 4. Use of drugs and medications; 5. Systemic diseases | Management was guided by Brain Trauma Foundation guidelines 2007 | At admission | Medical database | Mortality at 14 days and 6 months; GOSE at 6 months | Correlation with IMPACT and CRASH | 6 months |
| Le Bail et al., 2021[25] |
France | Retrospective observational study | To assess the predictive value of NLR I predicting neurological deterioration in mild and moderate TBI | n=115 | 2017–2020 | Adult TBI patients with brain contusion in single center | 1. Adult; 2. GCS≥10 | 1. Admission>24 h after trauma; 2. Mechanical ventilation; 3. Discharge<48 h after admission; 4. Chronic blood disease affecting NLR | Not reported | At admission | Medical database | Delayed clinical deterioration (GCS<10) or mechanical ventilation | In-hospital mortality, length of hospital stay>10 days, and modalities of hospital discharge | 5 days |
| Xie et al., 2021[19] |
China | Retrospective observational study | To assess the relationship between NLR and GCS with 6 months outcome of DAI patients | n=93 | January 2014–January 2020 | Adult DAI patients attending single institution | DAI patients | 1. Admission after 24 h of injury; 2. Expired or referred to other hospital; 3. CT/MRI showing EDH/SDH; 4. Concomitant multiple organ injury/surgery; 5. Steroids/immunosuppressants; 6. Blood collected 24 h after admission; 7. Infectious disease within 1 week of admission | Not reported | At admission | Medical database | GOS | Age, GCS | 6 months |
TBI: Traumatic brain injury, NLR: Neutrophil-lymphocyte ratio, CT: Computed tomography, GOS: Glasgow outcome scale, GOSE: Extended Glasgow outcome scale
| Study Id | Results | Key Conclusions | Remarks |
|---|---|---|---|
| Chen et al., 2018[24] |
NLR was found as significant predictor for unfavorable outcome (OR=1.100, 95% CI=1.064–1.138) and mortality (OR=1.158, 95% CI=1.094–1.226); mean NLR in favorable versus unfavorable outcome was (11.60±4.05 vs. 15.07±6.63) and mortality was 13.75±6.27 versus 18.75±7.76; predictive performance was similar to GCS in severe TBI for functional outcome and worse than GCS for mortality | Increased NLR at admission in severe TBI patients is associated with poor functional outcome and mortality at 1 year | Only adult patients with GCS≤8 within 6 h of admission were included in the study. Correlation of day 1 NLR at admission was done with functional outcome and mortality but not with the length of hospital stay, ventilator days, and GCS |
| Chen et al., 2019[9] |
Age, GCS, surgery in the first 24 h, length of hospital stay, and peak NLR were significantly associated with unfavorable outcome with OR 1.086 (95% CI 1.037–1.137); peak NLR cutoff value of 18.16 with sensitivity of 74.3% and specificity of 72.9%. NLR peaked between day 2 and day 4 | Day 1 NLR was associated with unfavorable outcome; however, peak NLR was significantly associated with unfavorable outcome after multivariate analysis. Day 1 NLR and GCS were associated with peak NLR in patients with severe TBI | Only adult patients with GCS≤8 within 24 h of admission were included in the study. Correlation of day 1 NLR at admission was done with functional outcome but not with the length of hospital stay, ventilator days, GCS, and mortality. The study showed that day 1 NLR was associated with peak NLR>21 and unfavorable outcome but peak NLR is better prognostic indicator than day 1 NLR. |
| Corbett et al., 2019[10] |
NLR (AUROC 0.500, 95% CI 0.442–0.559;P=0.998) was not a significant predictor of unfavorable outcome at 18 months in univariate or multivariate analysis | INR in isolation had the best prognostic significance in functional outcome of severe TBI patients requiring decompressive craniectomy. However, none of the hematological parameters including INR and NLR was a significant predictor of unfavorable outcome at 18 months or added additional prognostic value to IMPACT prognostic model | The study included adult patients with severe TBI requiring decompressive craniectomy and focused on abnormal hematological parameters in predicting unfavorable outcome at 18 months. Single NLR value was assessed. There was no distinction based on time of admission and correlation with admission GCS. Outcome assessed was GOS at 18 months and mortality was not reported separately. |
| Siwicka-Gieroba et al., 2019[8] |
Median NLR at admission was 11.74, highest was in patients with diffuse axonal injury. NLR was significantly higher in GOSE 1, 2, and 3, and cutoff value of 15.63 was associated with significant increase in 28 days mortality risk | NLR is a significant marker of outcome after severe TBI. Higher values of admission NLR and NLR in the 1stweek were associated with severe disability in TBI patients | The admission and 1stweek NLR were correlated with the GOSE outcome at 6 months and according to the TBI type. No association with surgery, length of hospital stay, ventilator status, and mortality were reported. |
| Zhao et al., 2019[22] |
NLR was significant predictor of 6-month functional outcome with OR 0.91 (95% CI; 0.89–0.93). Other significant predictors were age, admission GCS, coagulopathy, SDH, IPH, and tSAH | High day 1 NLR was a significant predictor of poor functional outcome at 6 months following TBI | Correlation of day 1 NLR at admission was done with functional outcome but not with the mortality, TBI types, length of hospital stay, ventilator days, and GCS. This study was not on isolated TBI and mean GCS was 11.21±3.70. |
| Dolmans et al., 2020[21] |
No laboratory parameter was associated with length of hospital stay more than 30 days, mortality, and functional outcome at 3 months | Routine blood investigations do not predict the length of hospital stay, 30-day mortality, and 3 months functional outcome in severe TBI patients | The study described initial laboratory values in patients with severe TBI and reported the correlation with outcomes measures as OR; however, data not presented in each arm of the groups compared. |
| Kim et al., 2020[20] |
Age, GCS, Cr level, aPTT, intraoperative epinephrine, and lymphocyte count (HR=1.085, 95% CI=1.006–1.169) were significant predictors of 1-year mortality. NLR was lower among the survivors and was not a significant predictor of mortality. | Prolonged aPPT, low GCS, and increased admission lymphocyte counts were associated with higher mortality at 1 year after emergency craniectomy for EDH and SDH | The study assessed mortality at 1 year in post-surgery patients. Only EDH and SDH were included in the study. There was no separate classification as per severity of injury. Other outcome measures including functional outcome were not reported. |
| Kimball et al., 2020[26] |
NLR was higher in patients with LOC, no significant relation with PTA and GCS. NLR at 24 h and 8 h was significantly different for different GOSE, but admission NLR and 72 h NLR were not significantly different. A 24 h and 48 h NLR were higher in patients who did not survived | Higher NLR at day 1 and day 2 was associated with worse outcomes in pediatric TBI | Data on NLR were not reported for survivors versus non-survivors. Data on outcome dichotomized as favorable and non-favorable were not present. |
| Mukherjee et al., 2020[23] |
NLR was independent predictor of outcome in pediatric TBI (OR2.61, 95% CI 1.30–7.99). NLR cutoff of 5.2 was a significant predictor for unfavorable outcome. | NLR is an independent risk factor for poor outcome in pediatric TBI patients | The study did not reported data among survivors versus non-survivors and outcome measure used was PCPCS and not GOSE-pediatric score. |
| Bilgi et al., 2021[11] |
TLC more than 20.95×106/L predicted mortality with 80% specificity and 50% sensitivity. Admission NLR was not a significant predictor of mortality or 6 months functional outcome | INR, TLC, and blood transfusion were significant predictor of mortality and 6 months functional outcome, whereas NLR was not a significant predictor | Data no reported separately for survivors versus non-survivors |
| Le Bail et al., 2021[25] |
Higher NLR at admission was associated with neurological deterioration (18 [12–29] vs. 8 [5–13]. NLR≥15), the sensitivity and specificity were 69% and 79% | NLR at admission was an independent predictor of neurological deterioration in mild or moderate TBI | Data on NLR and functional outcome were not reported. |
| Xie et al., 2021[19] |
NLR was an independent risk factor for 6-month unfavorable outcome in diffuse axonal injury with (OR: 1.63; 95% CI: 1.222e2.129). NLR above 14.99 had sensitivity and specificity of 80.6% and 94.7% in differentiating favorable from unfavorable outcome | NLR is an independent risk factor for poor outcome and NLR with GCS is a better indicator than the NLR or GCS alone | Data on mortality not presented separately |
TBI: Traumatic brain injury, NLR: Neutrophil-lymphocyte ratio, CT: Computed tomography, GOS: Glasgow Outcome Scale, GOSE: Extended Glasgow Outcome Scale, TLC: Total leukocyte count
| Study ID | Adult | Pediatric | Isolated TBI | Severe TBI | Moderate TBI | Mild TBI | Day 1 NLR | Serial NLR | Mortality | GOS | GOS-E | Pediatric GOSE | Other outcome scale | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. 2018[24] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 1 year | |||||||
| Chen et al. 2019[9] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 1 year | |||||||
| Corbett et al. 2019[10] | ✓ | ✓ | ✓ | ✓ | ✓ | 18 months | ||||||||
| Siwicka et al. 2019[8] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 months | |||||||
| Zhao et al. 2019[22] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 months | |||||||
| Dolmans et al. 2020[21] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Length of hospital days more than 30 days | 3 months | ||||||
| Kim et al. 2020[20] | ✓ | ✓ | ✓ | ✓ | ✓ | 1 year | ||||||||
| Kimball et al. 2020[26] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 86 days | ||||
| Mukherjee et al. 2020[23] |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Length of hospital stay and pediatric cerebral performance category scale score (PCPCS) | 6 months | ||||||
| Bilgi et al. 2021[11] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 14 day mortality | 6 months | ||||
| Le Bail et al. 2021[25] | ✓ | ✓ | ✓ | ✓ | ✓ | Neurological deterioration, length of hospital stay | 5 days | |||||||
| Xie et al. 2021[19] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 months |
TBI: Traumatic brain injury, NLR: Neutrophil-to-lymphocyte ratio, GOS: Glasgow outcome scale, GOSE: Extended Glasgow outcome scale
Quality assessment and risk of bias
All studies were of good quality as per the NOS with a score ≥6. The NOS quality assessment is shown in [Table 6]. The median NOS score of the studies was 8 with nine studies[8-11,19,20,22-24] scoring 8/9 and three studies[21,25,26] scoring 6/9 with a median score of 8. RoBANS risk of bias assessment is shown in [Figure 2]. There was a higher risk of detection bias in all the studies as there was no blinding reported in the outcome assessment and a low risk of reporting and performance bias. One study[22] had an unclear risk of selection bias while the rest of the studies had a low risk of selection bias. All studies had low risk of confounding bias.
| Groups | Selection | Comparability | Outcome | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Study Id | Representativeness of sample | Selection of the non-exposed cohort | Ascertainment of prognostic variable | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | |
| Chen et al., 2018[24] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 |
| Chen et al., 2019[9] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 |
| Corbett et al., 2019[10] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 |
| Siwicka-Gieroba et al., 2019[8] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 |
| Zhao et al., 2019[22] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 | |
| Dolmans et al., 2020[21] | ★ | ★ | ★ | ★ | ★ | ★ | 6/9 | ||
| Kim et al., 2020[20] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 |
| Kimball et al., 2020[26] | ★ | ★ | ★ | ★ | ★ | ★ | 6/9 | ||
| Mukherjee et al., 2020[23] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 |
| Bilgi et al., 2021[11] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 |
| Le Bail et al., 2021[25] | ★ | ★ | ★ | ★ | ★ | ★ | 6/9 | ||
| Xie et al., 2021[19] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 |
★Indicates that it meets criteria in Newcastle-Ottawa Scale

- (a) Risk of bias assessment in included studies using RoBANS. A summary table of review authors’ judgments for each risk of bias item for each study, (b) RoBANS risk of bias graph: Review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Results of syntheses
Seven studies were eligible for result synthesis for the predictive role of NLR in predicting GOS at a minimum follow-up of 6 months after TBI.[8-11,19,22,24] All these studies had isolated severe TBI patients and reported day 1 NLR. Four of these studies reported an association between higher admission NLR with an increased risk of unfavorable outcome.[9,19,22,24] Three studies found that the effect estimate overlapped the null line with no significant association between the admission NLR and GOS outcome.[8,10,11] Three studies had follow ups for ≥ 12 months. A minimum follow-up of 6 months was reported in four studies. The total number of participants in the quantitative synthesis was 2940. A meta-analysis showed that the mean difference was −5.18 (95% confidence interval: −10.04, −0.32). The results were statistically significant, with an overall effect of Z = 2.09 and P = 0.04. However, there was a high heterogeneity (I2) of 98%. This heterogeneity could be due to the difference in the study participants and the follow-up duration. Accordingly, we did a sensitivity analysis to address the heterogeneity. Even with the sensitivity analysis, the heterogeneity was high. The results were not significant for the studies with 6-month follow-ups. However, the results were significant for the studies with follow-ups of more than 6 months with an effect estimate of mean difference of −2.89 (95% confidence interval: −5.96, 0.17) and P = 0.06. The forest plot is shown in [Figure 3].

- Forest plot of neutrophil-to-lymphocyte ratio and Glasgow Outcome Scale outcome.
Two studies qualified for result syntheses for mortality as only two reported the data of survivors versus non-survivors.[20,24] The results showed that a higher admission NLR was associated with an increased mortality risk; however, the results were not significant. The number of participants for this group was 888. The effect estimate was a mean difference of −3.22 (95% confidence interval: −7.12, 0.68), P = 0.11, and I2 85%. The forest plot of the analysis is shown in [Figure 4]. The funnel plot for the synthesis of the results is shown in [Figure 5]. Meta-analysis for AUC receiver operating characteristic (ROC) of the included studies showed good predictive power of NLR in predicting outcomes following TBI with AUC 0.706 (95% CI: 0.582–0.829). The results for different studies with 95% CI and the pooled area under ROC in fixed-effect and random-effect models with 95% CI are shown in [Figure 6]. [Table 7] details the statistical methods and effect estimates of result syntheses and sensitivity analysis.
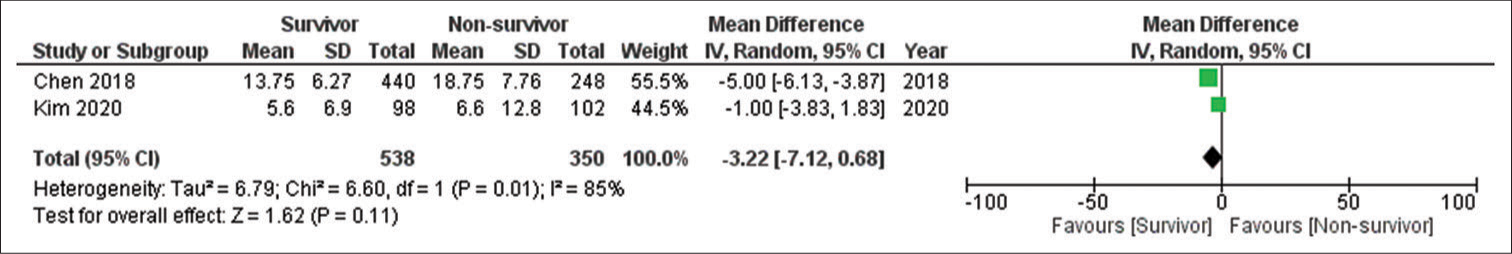
- Forest plot of neutrophil-to-lymphocyte ratio and mortality outcome.
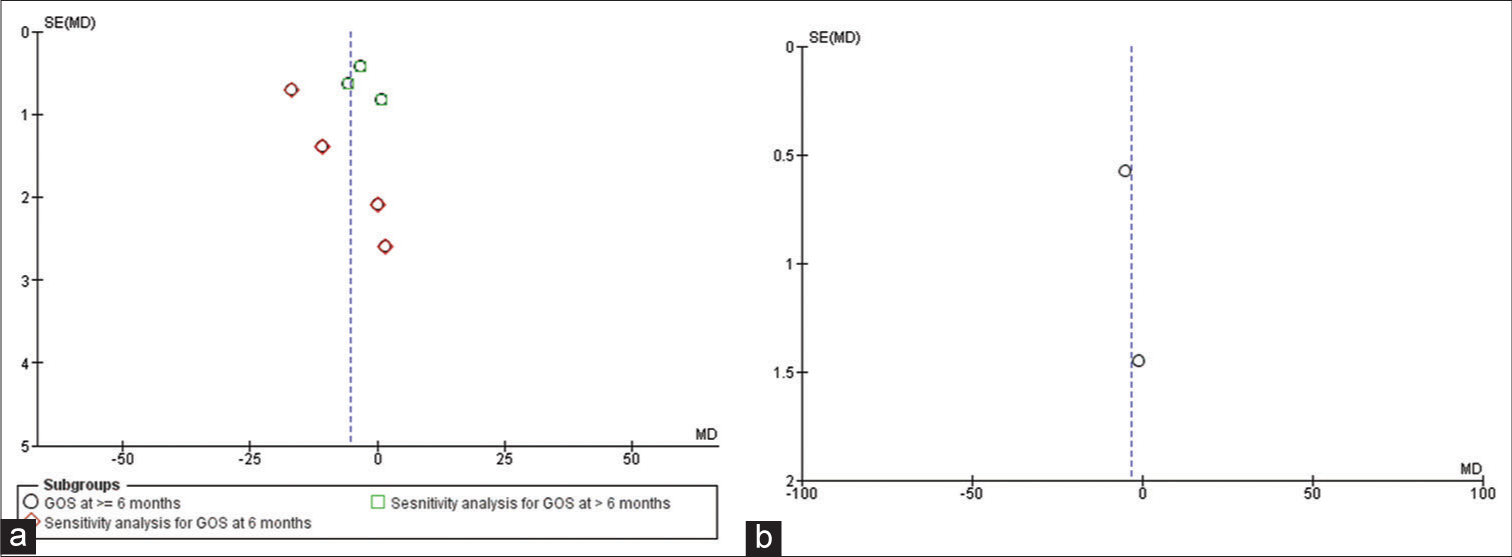
- (a) Funnel plot for studies reporting Glasgow Outcome Scale outcome, (b) funnel plot for studies reporting mortality outcome.
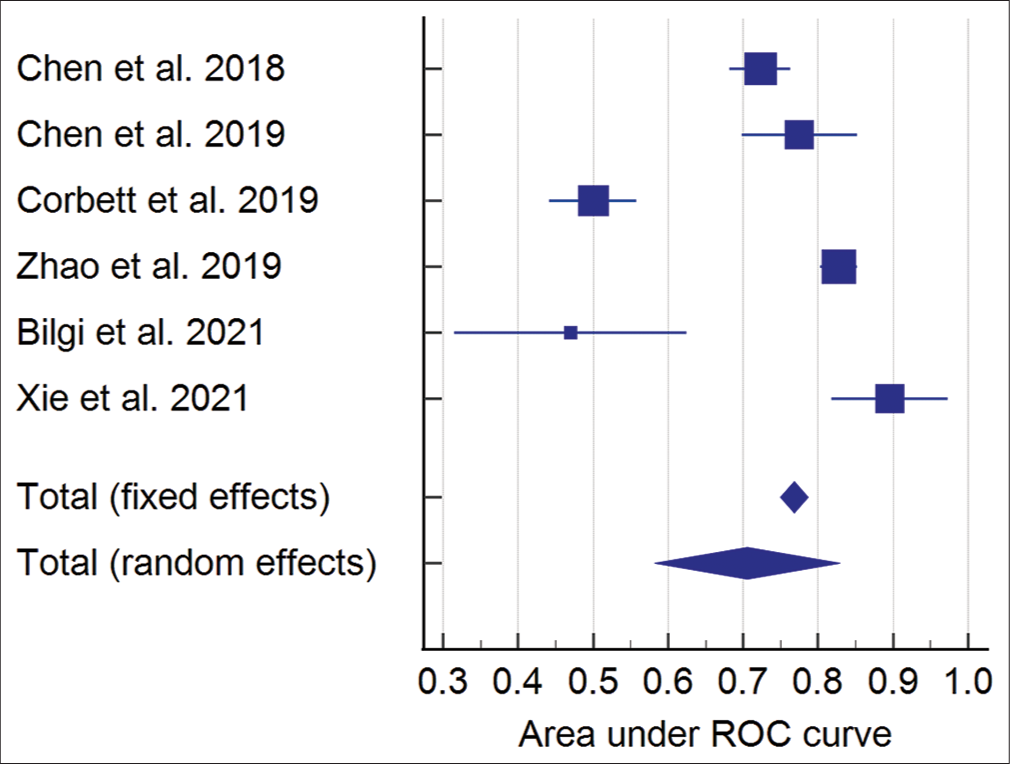
- Meta-analysis of AUC ROC for neutrophil-to-lymphocyte ratio predicting Glasgow Outcome Scale outcome.
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
|---|---|---|---|---|
| 1.1. GOS | 7 | 5880 | Mean difference (IV, random, 95% CI) |
−5.21 [−8.35, −2.06] |
| 1.1.1. GOS at≥6 months | 7 | 2940 | Mean difference (IV, random, 95% CI) |
−5.18 [−10.04, −0.32] |
| 1.1.2. Sensitivity analysis for GOS at 6 months | 4 | 1548 | Mean difference (IV, random, 95% CI) |
−6.89 [−15.35, 1.58] |
| 1.1.3. Sensitivity analysis for GOS at>6 months | 3 | 1392 | Mean difference (IV, random, 95% CI) |
−2.89 [−5.96, 0.17] |
| 1.2. Mortality | 2 | 888 | Mean difference (IV, random, 95% CI) |
−3.22 [−7.12, 0.68] |
GOS: Glasgow Outcome Scale, NLR: Neutrophil-to-lymphocyte ratio
DISCUSSION
The present systematic review evaluated the available evidence on the prognostic role of predicting admission NLR in predicting outcomes following TBI. Twelve studies were included for the qualitative synthesis. The outcome measures for the quantitative synthesis were GOS and mortality. A limited number of studies reported on other outcome measures of length of hospital stay and intensive care unit (ICU) stay, and therefore, the meta-analysis could not be done. NLR as a prognostic indicator of functional outcome after TBI is complex. Most published studies reported day 1 NLR as a predictor of outcome, while NLR is a dynamic entity. Chen et al.[9] showed that day 1 NLR was significantly associated with peak NLR >21 in patients with severe TBI. NLR peaked between day 2 and day 4. However, researchers found that only peak NLR was a significant predictor in multivariate analysis. This implies that day 1 NLR, though it predicts a peak rise in NLR, is peak NLR which is a better prognostic indicator.
Significant heterogeneity was observed among the included studies in terms of the follow-up. The studies are limited to severe TBI, and only one study focused on delayed deterioration in patients with TBI with GCS >10.[20] Most of the studies involved adult patients – two studies reported in the pediatric age group.[23,26] Zhao et al.[22] reported that the predictive value of NLR was better when used in the model along with other predictive parameters than with the NLR being used alone.
The present systematic review found that the admission NLR predicts the GOS with statistical significance. Higher NLR was associated with an increased risk of unfavorable outcomes at 6 months and more than 12 months follow-up. However, the certainty of the evidence was low due to high heterogeneity due to the changes in the study participants. The heterogeneity remained high in the sensitivity analysis, suggesting that the follow-up duration was not a factor responsible for high heterogeneity.
Studies have explored the role of neutrophils in the adverse outcomes following TBI. Neutrophils are present in circulation but not usually present in the brain parenchyma due to the blood–brain barrier.[7] Limited neutrophils are present in cerebrospinal fluid, pia, and meninges; however, pathological invasion of neutrophils in the brain parenchyma occurs in trauma, infection, ischemia, and hemorrhage.[27] Neutrophils result in tissue damage by phagocytosis, degranulation, and neutrophil extracellular trap. The accumulation of neutrophils is mediated by several receptors signaling the danger signal. Neutrophils can augment autocrine-dependent activation even when the danger signal has passed.[28-30] This led to indiscriminate tissue damage and neutrophils and was stopped by macrophages and lymphocytes. A similar mechanism is thought to activate after the trauma with the invasion and activation of neutrophils in the damaged brain due to TBI. The brain is a privileged immune organ due to the blood–brain barrier. However, there is invasion and instant activation of microglia and neutrophils in the damaged brain.[2,27,31,32] Recently discovered lymphatic channels lining the dural sinuses with characteristic lymphatic endothelial lining showed that once thought immune privilege status of the brain is changed. These channels provide a route for entry and exit of peripheral immune cells to the brain.[1,33] The primary injury sets the stage for secondary brain injury, resulting in edema and reduced cerebral blood flow. The shear stress results from the mechanical forces due to the primary impact disrupting axons and blood vessels. This results in cerebral edema, the release of inflammatory cytokines, disruption of the blood–brain barrier, neuroinflammation, and invasion of the peripheral immune system.[34] Animal and human studies showed that there is hypoperfusion in the early stages of TBI and results in poor neurological outcome.[35-38] This hypoperfusion results in activation and accumulation of neutrophils and the rheological action of neutrophils in blood vessels. Accordingly, increased local neutrophils result in indiscriminate brain damage. Researchers identified that this local increase in the neutrophil count at the damaged brain site is reflected in increased neutrophils in the peripheral blood. Several studies found that increased neutrophils after TBI and increased NLR predict poor functional outcome. A study by Bilgi et al.[11] reported that NLR was not superior to the CRASH and IMPACT scoring system in predicting mortality or functional outcome. Similarly, the study by Korobey et al.[12] reported that the predictive power of NLR was inferior to the CRASH predictive model, and no additional value was obtained when NLR was included in the predictive model system.
Clinical implications
NLR is a routine and straightforward investigation done in TBI patients. The NLR at admission is a simple biomarker for predicting the functional outcome (GOS) at 6 months. The predictive power of NLR is better when GOS is assessed at 12 months. However, the strength of the evidence available is low. The available evidence is for the adult population. TBI and inflammation are different in children as compared to adults. There is no evidence currently available to recommend NLR as a predictive biomarker of outcome following TBI in children.
Research implications
There was high heterogeneity among the available studies. The future studies focusing on the predictive value of NLR in children, predictive value according to the severity of TBI, and type of TBI will be more beneficial and informative to make clinical recommendations. Studies exploring other outcome measures, including length of hospital stay, ICU stay, ventilator days, and long-term functional outcomes, including cognitive function and long-term complications including neurocognitive sequelae and dementia, will be more meaningful. The strength of recommendations from this review is very low as the studies included were retrospective in nature, we recommend more high-end prospective research controlling the confounding factors in this topic.
Limitations
Most of the studies included in the present systematic review were retrospective studies and posed limitations in the strength of the evidence available. The heterogeneity in the participant characteristics in terms of types of TBI and severity of TBI is a significant limitation. Non-availability of comparison with standard prognostic indicators such as GCS limits the quality of available evidence. One of the limitations we faced which can influence the generalizability of these results is that the authors in the included studies did not mention the medications used in the pre-hospital treatment or intra-hospital treatment phase and this can affect the NLR in these patients. Although most of the studies mention including patients within 24 h of trauma and measuring day 1 NLR, there is variability among the studies about the time gap of collection of samples for NLR analysis from the trauma and this could affect the results.
CONCLUSION
NLR is a simple biomarker that is routinely performed in TBI patients and can significantly predict the outcome assessed by GOS at 6 months. High NLR is associated with an increased risk of unfavorable outcomes following TBI. There was no significant correlation between the NLR and mortality. The AUC ROC meta-analysis showed good predictive power of NLR in predicting GOS outcome following TBI with AUC 0.706 (95% CI: 0.582–0.829). The strength of evidence is low, making clinical recommendations of low strength to recommend using NLR as a stand-alone predictive tool in TBI patients.
Acknowledgments
We acknowledge the support of library of our institute.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Traumatic brain injury and peripheral immune suppression: Primer and prospectus. Front Neurol. 2015;6:235.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4-9.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropsychiatric complications of traumatic brain injury: A critical review of the literature (a report by the ANPA Committee on Research) J Neuropsychiatry Clin Neurosci. 2007;19:106-27.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of outcome after severe brain damage. Lancet. 1975;1:480-4.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of trauma on neutrophil function. Injury. 2014;45:1824-33.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378-88.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519-31.
- [CrossRef] [PubMed] [Google Scholar]
- The neutrophil/lymphocyte count ratio predicts mortality in severe traumatic brain injury patients. J Clin Med. 2019;8:E1453.
- [CrossRef] [PubMed] [Google Scholar]
- Peak neutrophilto-lymphocyte ratio correlates with clinical outcomes in patients with severe traumatic brain injury. Neurocrit Care. 2019;30:334-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of abnormal hematological parameters in severe traumatic brain injury requiring decompressive craniectomy. J Neurosurg. 2019;132:545-51.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome prediction of TBI: Are there parameters that affect the IMPACT and CRASH models? World Neurosurg. 2021;146:e590-6.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil/lymphocyte ratio and neurologic outcome in traumatic brain injury: Comparison to crash. Crit Care Med. 2020;48:848.
- [CrossRef] [Google Scholar]
- The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- [CrossRef] [PubMed] [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions. New York: John Wiley and Sons; 2019.
- [CrossRef] [Google Scholar]
- 2022. Available from: www.training.cochrane.org/handbook [Last accessed on 2022 Feb 05]
- [Google Scholar]
- Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29:2520-37.
- [CrossRef] [PubMed] [Google Scholar]
- Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-5.
- [CrossRef] [PubMed] [Google Scholar]
- Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408-14.
- [CrossRef] [PubMed] [Google Scholar]
- Combination of neutrophil-to-lymphocyte ratio and admission glasgow coma scale score is independent predictor of clinical outcome in diffuse axonal injury. World Neurosurg. 2021;152:e118-27.
- [CrossRef] [PubMed] [Google Scholar]
- Hematological factors predicting mortality in patients with traumatic epidural or subdural hematoma undergoing emergency surgical evacuation: A retrospective cohort study. Medicine (Baltimore). 2020;99:e22074.
- [CrossRef] [PubMed] [Google Scholar]
- Routine blood tests for severe traumatic brain injury: Can they predict outcomes? World Neurosurg. 2020;136:e60-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of neutrophil-to-lymphocyte ratio in predicting the 6-month outcome of patients with traumatic brain injury: A retrospective study. World Neurosurg. 2019;124:e411-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of leukocytosis in pediatric traumatic brain injury. J Neurosurg Pediatr. 2020;27:335-45.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil to lymphocyte ratio as a novel predictor of outcome in patients with severe traumatic brain injury. J Head Trauma Rehabil. 2018;33:E53-9.
- [CrossRef] [PubMed] [Google Scholar]
- Ability of neutrophil-to-lymphocyte ratio to predict secondary neurological impairment in patients with mild to moderate head injury. A retrospective study. Am J Emerg Med. 2021;50:46-50.
- [CrossRef] [PubMed] [Google Scholar]
- Using the neutrophil-to-lymphocyte ratio to predict outcomes in pediatric patients with traumatic brain injury. Clin Neurol Neurosurg. 2020;193:105772.
- [CrossRef] [PubMed] [Google Scholar]
- Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368-79.
- [CrossRef] [PubMed] [Google Scholar]
- Autocrine role of endogenous interleukin-18 on inflammatory cytokine generation by human neutrophils. FASEB J. 2009;23:194-203.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of leukotriene synthesis in human neutrophils by exogenous arachidonic acid: Inhibition by adenosine A(2a) receptor agonists and crucial role of autocrine activation by leukotriene B(4) Mol Pharmacol. 1999;56:1055-62.
- [CrossRef] [PubMed] [Google Scholar]
- Zymosan-induced IL-8 release from human neutrophils involves activation via the CD11b/ CD18 receptor and endogenous platelet-activating factor as an autocrine modulator. J Immunol. 1994;152:5411-9.
- [Google Scholar]
- Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337-41.
- [CrossRef] [PubMed] [Google Scholar]
- Traumatic brain injury: Recent advances in plasticity and regeneration. Curr Opin Neurol. 2015;28:565-73.
- [CrossRef] [PubMed] [Google Scholar]
- Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35-43.
- [CrossRef] [PubMed] [Google Scholar]
- Defining ischemic burden after traumatic brain injury using 15O PET imaging of cerebral physiology. J Cereb Blood Flow Metab. 2004;24:191-201.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in cerebral blood flow from the acute to the chronic phase of severe head injury. J Neurotrauma. 2005;22:1411-8.
- [CrossRef] [PubMed] [Google Scholar]
- Brief episodes of intracranial hypertension and cerebral hypoperfusion are associated with poor functional outcome after severe traumatic brain injury. J Trauma. 2011;71:364-73.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg. 1997;86:633-41.
- [CrossRef] [PubMed] [Google Scholar]
- Head trauma and leucocytosis. Acta Neurochir (Wien). 1994;131:211-4.
- [CrossRef] [PubMed] [Google Scholar]
- Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291-8.
- [CrossRef] [PubMed] [Google Scholar]
- The blood leukocyte count and its prognostic significance in severe head injury. Surg Neurol. 2001;55:190-6.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated head injury in children affects the neutrophil function and lymphocyte count. J Trauma. 2007;63:179-86.
- [CrossRef] [PubMed] [Google Scholar]
- CD64-Neutrophil expression and stress metabolic patterns in early sepsis and severe traumatic brain injury in children. BMC Pediatr. 2013;13:31.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative burst of circulating neutrophils following traumatic brain injury in human. PLoS One. 2013;8:e68963.
- [Google Scholar]
- Serial serum leukocyte apoptosis levels as predictors of outcome in acute traumatic brain injury. Biomed Res Int. 2014;2014:720870.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-lymphocyte ratio and perihematomal edema growth in intracerebral hemorrhage. Stroke. 2017;48:2589-92.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophils in traumatic brain injury (TBI): Friend or foe? J Neuroinflammation. 2018;15:146.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: A system review. Transl Stroke Res. 2019;10:137-45.
- [CrossRef] [PubMed] [Google Scholar]
- The immunological response to traumatic brain injury. J Neuroimmunol. 2019;332:112-25.
- [CrossRef] [PubMed] [Google Scholar]
- The emerging role of neutrophils as modifiers of recovery after traumatic injury to the developing brain. Exp Neurol. 2019;317:144-54.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-tolymphocyte ratio predicts hematoma growth in intracerebral hemorrhage. J Int Med Res. 2019;47:2970-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic impact of leukocytosis in intracerebral hemorrhage: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e16281.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil to lymphocyte ratio as a predictive biomarker for computed tomography scan use in mild traumatic brain injury. Biomark Med. 2020;14:1085-90.
- [CrossRef] [PubMed] [Google Scholar]
- Association between neutrophil to lymphocyte ratio and malignant brain edema in patients with large hemispheric infarction. Curr Neurovasc Res. 2020;17:429-36.
- [CrossRef] [PubMed] [Google Scholar]
- The predictive role of postoperative neutrophil to lymphocyte ratio for 30-day mortality after intracerebral hematoma evacuation. World Neurosurg. 2020;134:e631-5.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroinflammation mechanisms and phytotherapeutic intervention: A systematic review. ACS Chem Neurosci. 2020;11:3707-31.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as an inflammatory biomarker in predicting the severity of secondary brain injury: A review article. Open Access Maced J Med Sci. 2020;8:272-82.
- [CrossRef] [Google Scholar]
- Early elevated neutrophil-to-lymphocyte ratio associated with remote diffusion-weighted imaging lesions in acute intracerebral hemorrhage. CNS Neurosci Ther. 2020;26:430-7.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-to-lymphocyte ratio and traumatic brain injury: A review study. World Neurosurg. 2020;140:142-7.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting mortality in traumatic brain injury: Comparison of neutrophil/lymphocyte ratio and crash. Crit Care Med. 2020;48:172.
- [CrossRef] [Google Scholar]
- Assessment of peripheral blood cell inflammatory markers in patients with chronic subdural hematoma. Clin Neurol Neurosurg. 2020;191:105738.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of blood glucose and inflammation markers in pediatric head injuries. East J Med. 2021;26:67-74.
- [CrossRef] [Google Scholar]
- Neutrophil to lymphocyte ratio a novel prognostic marker following spontaneous intracerebral haemorrhage. Clin Neurol Neurosurg. 2021;200:106339.
- [CrossRef] [PubMed] [Google Scholar]
- Neutrophil-to-lymphocyte ratio as an independent predictor of in-hospital mortality in patients with acute intracerebral hemorrhage. Medicina (Kaunas). 2021;57:622.
- [CrossRef] [PubMed] [Google Scholar]







