Translate this page into:
Neuromelioidosis: A farmer’s bane
*Corresponding author: Sreehari Dinesh, Department of Neurology, Bharati Vidyapeeth (Deemed to be University) Medical College and Hospital, Pune, Maharashtra, India. sreehari4657@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Dinesh S, Gupta D, Gorthi S. Neuromelioidosis: A farmer’s bane. J Neurosci Rural Pract. 2024;15:521-3. doi: 10.25259/JNRP_31_2024
Dear Editor,
INTRODUCTION
Neuromelioidosis is a fatal infection caused by Burkholderia pseudomallei. It is a gram-negative bacillus that belongs to the burkholderia genus. The B. pseudomallei is a category B bioterrorism agent classified by the Centre for Disease Control.[1] Here we present an unusual case of neuromeliodosis with CNS involvement (meningitis, empyema, vasculitic infarcts); peripheral nervous system involvement (spinal cord involvement) as well as extra-neuronal involvement.
CASE REPORT
A 48-year-old male farmer had fever, altered sensorium, and vomiting for three days. He also developed asymmetric lower-limb weakness (left weaker than right) one day before presentation and new-onset generalized tonic-clonic seizures. Two months ago, he was admitted to another hospital with new-onset diabetes and diabetic ketoacidosis, Escherichia coli sepsis, acute kidney injury, hepatic dysfunction, and incidental Leptospira Immunoglobulin M positivity. There he was ventilated for four days.
On examination, he had high-grade fever 103 F, pallor, and was dehydrated. Glasgow Coma score was 13/15 (E4M6V3). He was disoriented to time and place but was obeying simple commands. Examination of cranial nerves was normal. The tone in all four limbs was increased with power 3/5 and brisk reflexes, bilateral plantar response was extensor. He had neck stiffness and tenderness over D2–D5 spinal was present.
Investigations showed elevated blood sugars 343 mg/dL with urine ketones present and high anion gap metabolic acidosis. His hemoglobin A1c was elevated at 13.4%. He had anemia with hemoglobin of 9.3 mg/dL; ESR was elevated >140 and human immunodeficiency virus, hepatitis B surface antigen, and hepatitis C virus were negative. He had hypoalbuminemia with serum albumin 2.2 mg/dL and hyperbilirubinemia 2.3 mg/dL. Renal functions were normal. Cerebrospinal fluid (CSF) analysis showed one cell with elevated proteins 103.5 g/dL and sugars 175 mg/dL (venous glucose 156 mg/dL). CSF GeneXpert and Biofire were negative.
He was admitted in intensive care unit, and diabetic ketoacidosis was treated with fluid resuscitation and insulin infusion. He was started on a meningitic dose of antibiotics. Magnetic resonance imaging (MRI) of brain revealed a small epidural collection in the left parieto-occipital region with restricted diffusion suggestive of empyema [Figure 1]. Pachymeningeal and leptomeningeal enhancement were also present. An MRI spine revealed infective spondylitis D2–D3 level with 4 mm posterior epidural collection from D1-D6 suggestive of a liquified abscess [Figure 2]. In view of D2– D3 spondylodiscitis, he underwent laminectomy, posterior spine fixation, and abscess drainage. On the third day of hospitalization, his blood culture grew B. pseudomallei [Figure 3]. Maldi time of flight (TOF) analysis – matrix-assisted laser desorption and ionization TOF mass spectrometry showed both Burkholderia mallei – 2.03 and B. pseudomallei – 1.76. Pus culture from operated site of spine also grew B. pseudomallei. In view of Burkholderia neuroencephalomyelitis, the patient was started on injection ceftazidime 2 g IV three times a day (TDS) and trimethoprim and sulfamethoxazole (160/800 mg) BD. Since fever persisted, antibiotics were upgraded to injection meropenem 1 g IV TDS and Bactrim double strength (DS). On day 10 of hospitalization, he developed septic arthritis of the right knee. Synovial fluid analysis revealed sugar – 98, proteins 1.1, cells 38,400, and pus culture – B. pseudomallei [Figure 4]. He underwent arthroscopic right knee surgery and lavage. Seven days later, he had a sudden drop in sensorium with high fever. The patient was re-intubated. Repeat MRI brain showed multiple infarcts. Given suspected infective endocarditis, 2D echocardiography was done which was normal. He developed septic shock, multiorgan failure and passed away despite all efforts.
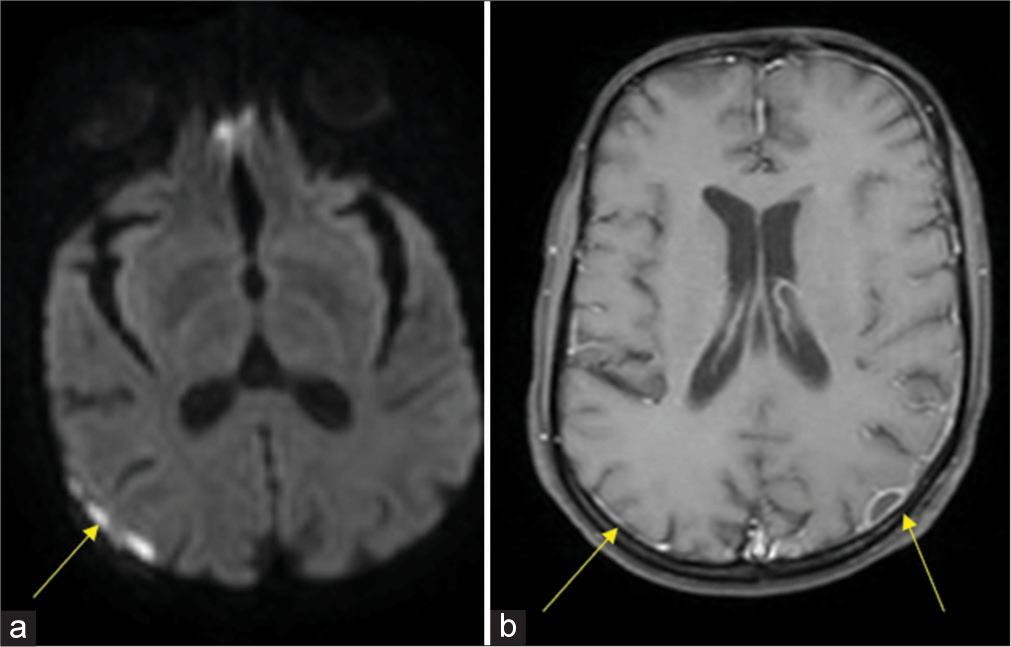
- Magnetic resonance imaging brain contrast: (a) Small epidural collection in left parieto-occipital region and right frontoparietal convexity with restricted diffusion suggestive of empyema (yellow arrow). (b) Pachymeningeal enhancement along left frontoparietooccipital convexity with leptomeningeal enhancement (yellow arrows).
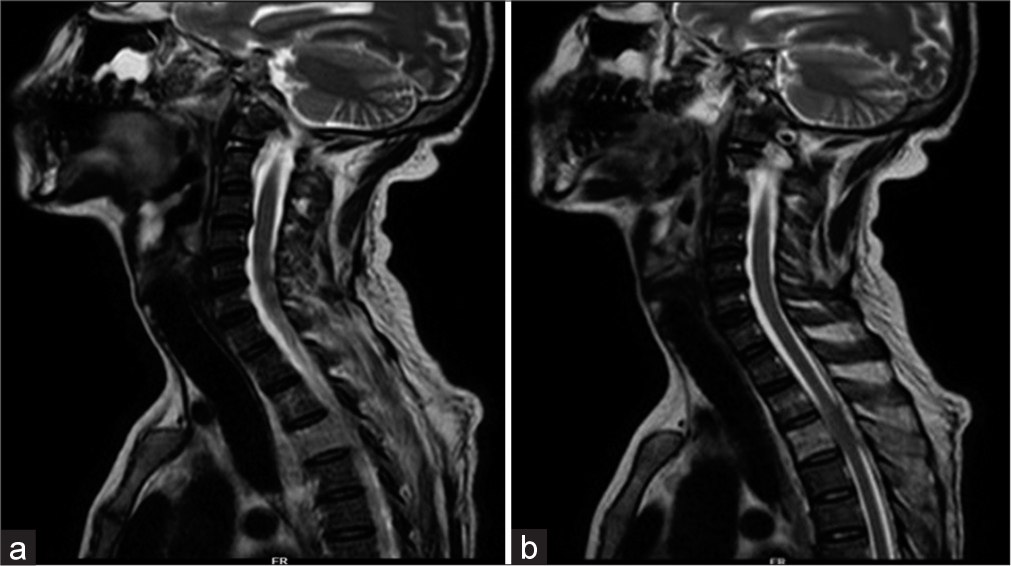
- (a) Magnetic resonance imaging (MRI) cervical and upper thoracic spine: Infective spondylitis D2–D3 level with large perivertebral soft tissue component. (b) MRI cervical and upper thoracic spine: Soft tissue around D3 showing peripheral enhancement suggestive of liquified abscess.
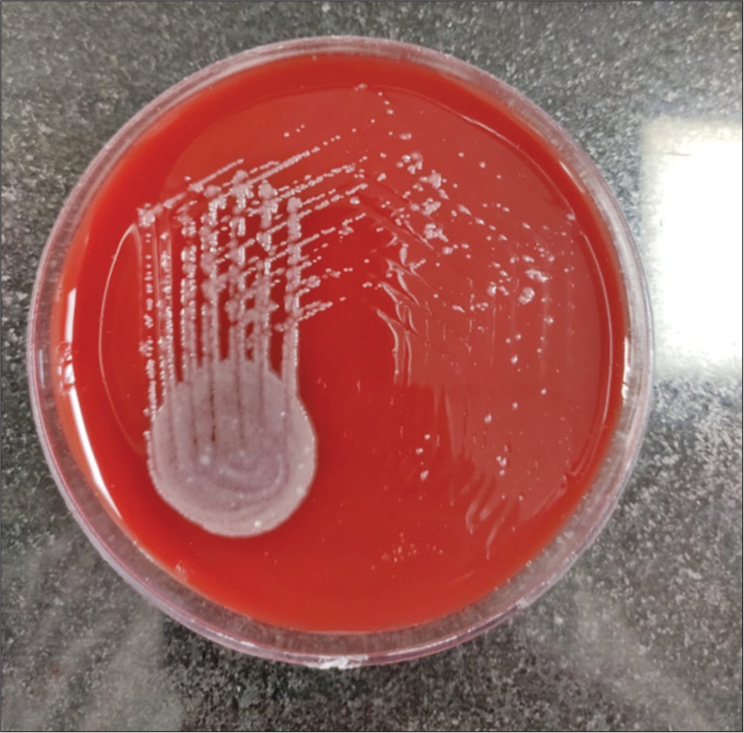
- Blood agar: Smooth creamy white colonies on ashdown medium for selective isolation.
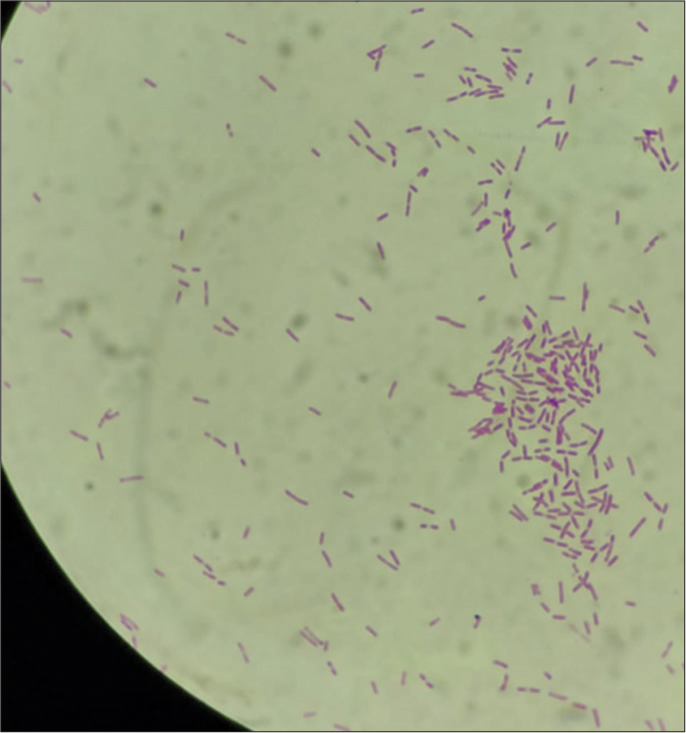
- Synovial fluid analysis: Gram-negative motile bacilli bipolar rod-shaped bacterium.
DISCUSSION
Melioidosis generally impacts humans in contact with dirty soil and water. Infection is through percutaneous inoculation.[2] Melioidosis is predominantly seasonal; about 80% cases occur during the monsoon and in floods.[3]
In a systematic review of 70 studies of central nervous system (CNS) melioidosis, 120 patients were studied. Most (70%) were males and the mean age was 40 years. Up to 60% had one or more risk factors for melioidosis. In addition to CNS involvement, 67% had at least one extra-neurological organ involvement. In patients with encephalitis and brain abscess, ring enhancement pattern was most common (78%). Mortality was 20%.[4]
A case series from Vellore South India described 18 cases of neuromelioidosis from 2008 to 2019. CNS involvement included parenchymal abscesses, focal cerebritis/encephalitis, and extradural and dural disease while spinal disease was classified into acute myelitis and spondylodiscitis. Extradural involvement included abscesses or thick irregular enhancement. In this case series, out of 18, 3 succumbed.[5]
Risk factors for neuromelioidosis are diabetes, chronic renal and lung disease, alcohol abuse, and immunosuppression. Neuromelioidosis is commonly diagnosed within 2 weeks after the first presentation. A 3–4-week intensive course of intravenous ceftazidime or meropenem therapy, followed by 3–6 months of trimethoprim and sulfamethoxazole orally for eradication therapy is recommended. Oral doxycycline can also be used after hospitalization.[6]
A similar case has been described in a 45-year-old man with diabetes mellitus and chronic liver cirrhosis. He had pneumonia and developed altered sensorium with CN 6 and 7 palsy and flaccid paralysis. Blood and urine culture grew B. pseudomallei. He succumbed despite appropriate antibiotics.[7]
A 32-year-old zoo worker presented with fever followed by flaccid areflexic quadriparesis and respiratory distress. Initially, he was thought to have Guillain-Barre syndrome (GBS). However, MRI brain showed multiple abscesses with ring-enhancing lesion in the brainstem and upper cervical cord. Chest imaging showed bilateral lung lower zone consolidation with cavity. Bronchoalveolar lavage grew Burkholderia pseudomallei. He was treated with meropenem. He survived with severe deficits.[8]
Nagendra and Shah have reported a 56-year-old lady with diabetes who had pachymeningitis with focal encephalitis secondary to neuromeliodosis and developed septic arthritis of knee joint, similar to our patient.[9]
CONCLUSION
Neuromelioidosis is a rare presentation of melioidosis. As shown in this case report, multiple sites in the neuroaxis as well as extraneural manifestations can occur in the same patient. Treatment consists of an initiation phase and a maintenance phase. Neuromeliodosis has a high mortality rate despite all efforts.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Melioidosis: Epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383-416.
- [CrossRef] [PubMed] [Google Scholar]
- Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2010;82:1113-7.
- [CrossRef] [PubMed] [Google Scholar]
- The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900.
- [CrossRef] [PubMed] [Google Scholar]
- Central nervous system melioidosis: A systematic review of individual participant data of case reports and case series. PLoS Negl Trop Dis. 2019;13:e0007320.
- [CrossRef] [PubMed] [Google Scholar]
- Neuromelioidosis: A single-center experience with emphasis on imaging. Indian J Radiol Imaging. 2021;31:57-64.
- [Google Scholar]
- Epidemiology of neuromelioidosis in Asia-pacific: A systematic review. Open Access Maced J Med Sci. 2021;9:318-26.
- [CrossRef] [Google Scholar]
- A melioidosis patient presenting with brainstem signs in the emergency department. J Emerg Med. 2013;44:e9-12.
- [CrossRef] [PubMed] [Google Scholar]
- Central and peripheral nervous system involvement in neuromelioidosis. Case Rep. 2015;2015:bcr2015211001.
- [CrossRef] [PubMed] [Google Scholar]
- Neuromeliodosis-a tropical illness presenting as meningoencephalitis with unusual brain imaging. Ann Indian Acad Neurol. 2021;24:97-8.
- [CrossRef] [PubMed] [Google Scholar]






