Translate this page into:
Neuroborreliosis: Unusual clinical presentation and imaging features
*Corresponding author: Manshi Kashyap, Department of Neurology, AIIMS, Bhopal, Madhya Pradesh, India. kashyapmansi58@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kashyap M, Kashyap PV. Neuroborreliosis: Unusual clinical presentation and imaging features. J Neurosci Rural Pract 2023;14:132-6.
Abstract
Lyme disease is a tick-borne infectious disease caused by Borrelia burgdorferi which causes a multi-organ involvement. It is endemic in North America and Europe, but not very commonly seen in India. Neurological manifestations (Lyme’s Neuroborreliosis,) can occur in both the early and late disseminated stages, and the classic triad consists of aseptic meningitis, painful radiculoneuritis, and cranial neuropathy. If untreated, it can be fatal and may lead to significant morbidity. We report a case with neuroborreliosis who developed acute onset and rapidly progressive bilateral vision loss, and we also report characteristic features on neuroimaging, including a characteristic “rounded M sign.” This unusual presentation, along with the characteristic imaging features, should be borne in mind to avoid misdiagnosis.
Keywords
Neuroinfectiology
Neuroborreliosis
Neurology
Tropical neurology
INTRODUCTION
Neurological manifestations of Lyme’s disease most commonly include radiculitis and cranial nerve palsies. Although papilledema due to raised intracranial pressure has been reported before, papillitis has seldom been reported. We report a case with neuroborreliosis who developed acute onset and rapidly progressive bilateral vision loss, and we also report characteristic features on neuroimaging, including a characteristic “rounded M sign’. The unusual clinical features in our case certainly expand the spectrum of neuroborreliosis. In addition, the peculiar imaging features would also help a clinician to make quicker diagnosis.
CASE REPORT
A 17-year-old girl presented with acute severe holocranial headache for 3 weeks, and loss of vision for 12 days. Loss of vision was acute, bilateral, and painless, which rapidly progressed to complete loss of light perception within 2–3 days. She also had myalgias, but did not have a fever. Her pupils were sluggishly reactive to light. There was bilateral lateral rectus palsy and signs of meningeal irritation. Slit-lamp examination was normal, while fundus examination revealed signs of papillitis. Her cerebrospinal fluid (CSF) analysis was inflammatory (Cells-35, 95% lymphomononuclear cells, protein−76 mg/ dL, glucose−54 mg/dL, and corresponding blood glucose: 102 mg/dL). CSF India ink staining, cryptococcal antigen, CBNAAT for M. tuberculosis, free living microorganism, Gram stain, and microbial cultures were negative. Magnetic resonance (MR) imaging of brain revealed minimal leptomeningeal enhancement in bilateral parafalcine region, and minimal exudates anterior to the midbrain [Figure 1a-c], while spinal cord imaging was normal. Serology for HIV, VDRL aquaporin-4 antibody, ANA, ANCA, and ACE levels were non-contributory. Based on existing evidences, and high prevalence of tuberculosis in India, anti-tubercular therapy (except ethambutol) with oral corticosteroid was started.
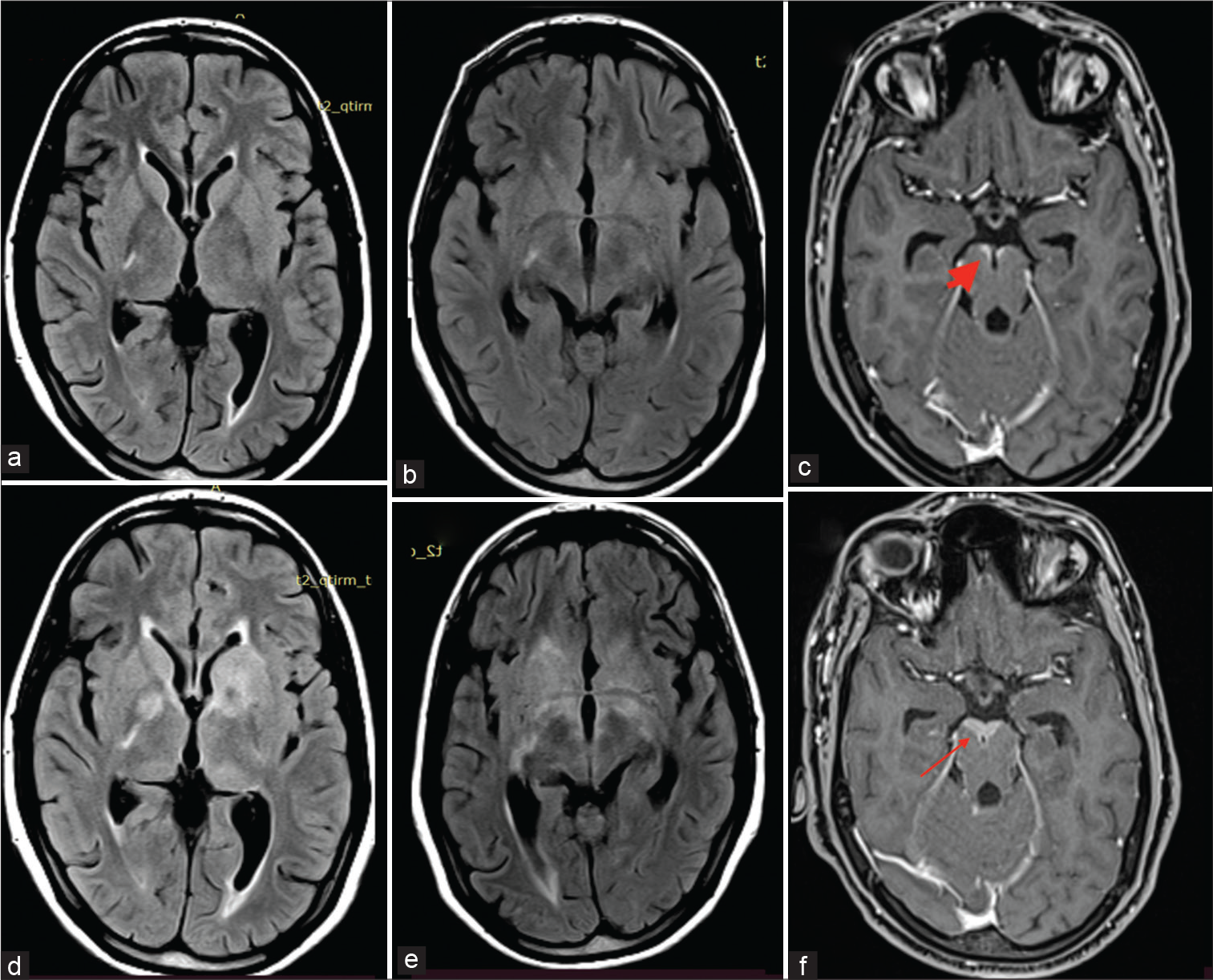
- (a-f) The first neuroimaging (FLAIR a and b) does not reveal any significant signal changes. Note is made of contrast enhancement anterior to the midbrain on T1 post-contrast (c) (thick arrow). Second neuroimaging (FLAIR d and e) shows extensive hyperintensities in bilateral basal ganglia region. There is also increase in the contrast enhancement in the midbrain on T1 post-contrast (f) (thin arrow). FLAIR: Fluid-attenuated inversion recovery.
However, on follow-up visit 2 weeks later, the patient had deteriorated with onset of seizures, left facial palsy, extremely severe radicular pains in both lower limbs, urinary retention, and arthritis involving predominantly large joints. Thereafter, she also developed bilateral sensorineural hearing loss. Her serum lactate level was normal. Repeated ophthalmological evaluation did not reveal ocular inflammatory changes. There was no effusion in the symptomatic Joints. CSF analysis was repeated which yielded similar results. Repeated MR neuroimaging showed a marked increase in the radiological features ([Figure 1a-c] represents first neuroimaging, while [Figure 1d-f] represents the second neuroimaging). There was an acute infarct in left corona radiata [Figure 1d], T2-weighted and fluid-attenuated inversion recovery (FLAIR) hyperintensities in bilateral basal ganglia [Figure 1d and e], along with meningeal enhancement in bilateral parafalcine, frontoparietal lobes, bilateral sylvian fissures, basal cisterns, bilateral cerebellar folia, interpeduncular cistern, and along multiple cranial nerves [Figure 2]. There was thin linear post-contrast leptomeningeal enhancement involving entire spinal cord and nodular enhancement in D12–L1 vertebral level [Figure 3]. Most interestingly, she had a rounded M pattern of contrast enhancement in the brainstem [Figures 3]. Having a strong suspicion for Lyme’s Neuroborreliosis (LNB), enzyme immunoassay for both IgM and IgG was sent which came out to be positive.
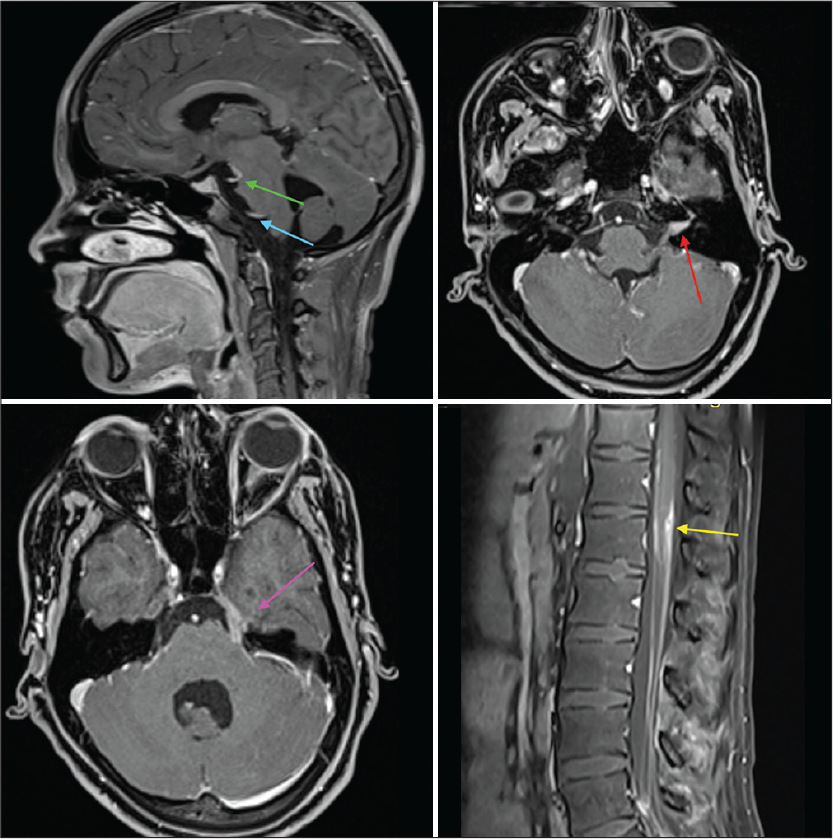
- T1 post-contrast images. Green and blue arrows show 3rd and 6th cranial nerve enhancement, respectively; red arrow shows enhancement of 7th and 8th cranial nerves in the left internal auditory meatus. Pink arrow shows contrast enhancement of 5th cranial nerve, while the yellow arrow shows nodular contrast enhancement in D12–L1 region.
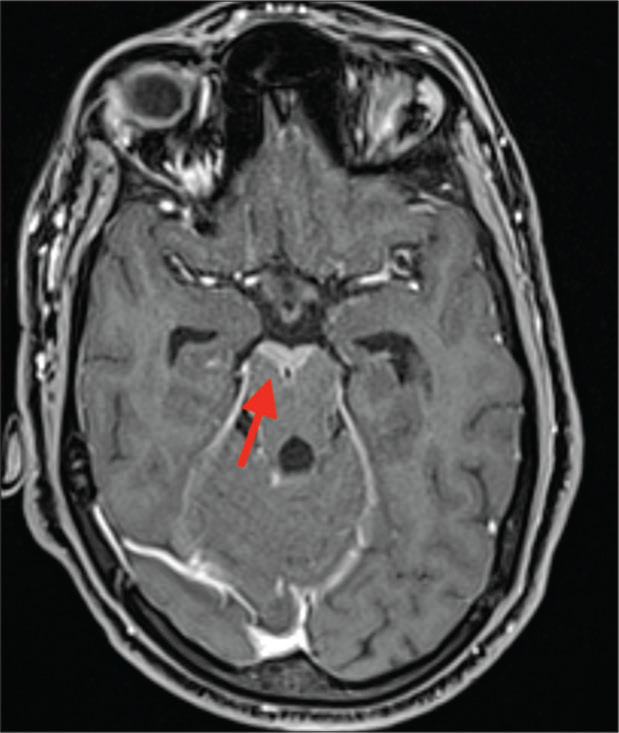
- T1 Post-contrast image showing contrast enhancement anterior to the midbrain (red arrow).
Ceftriaxone and doxycycline were started, and thereafter, the patient showed marked clinical improvement. Antibiotics were continued for 6 weeks in view of extensive neurological involvement. There was reduction in radiological abnormalities as well [Figure 4]. However, optic atrophy had already occurred, and she remained with residual visual deficit.
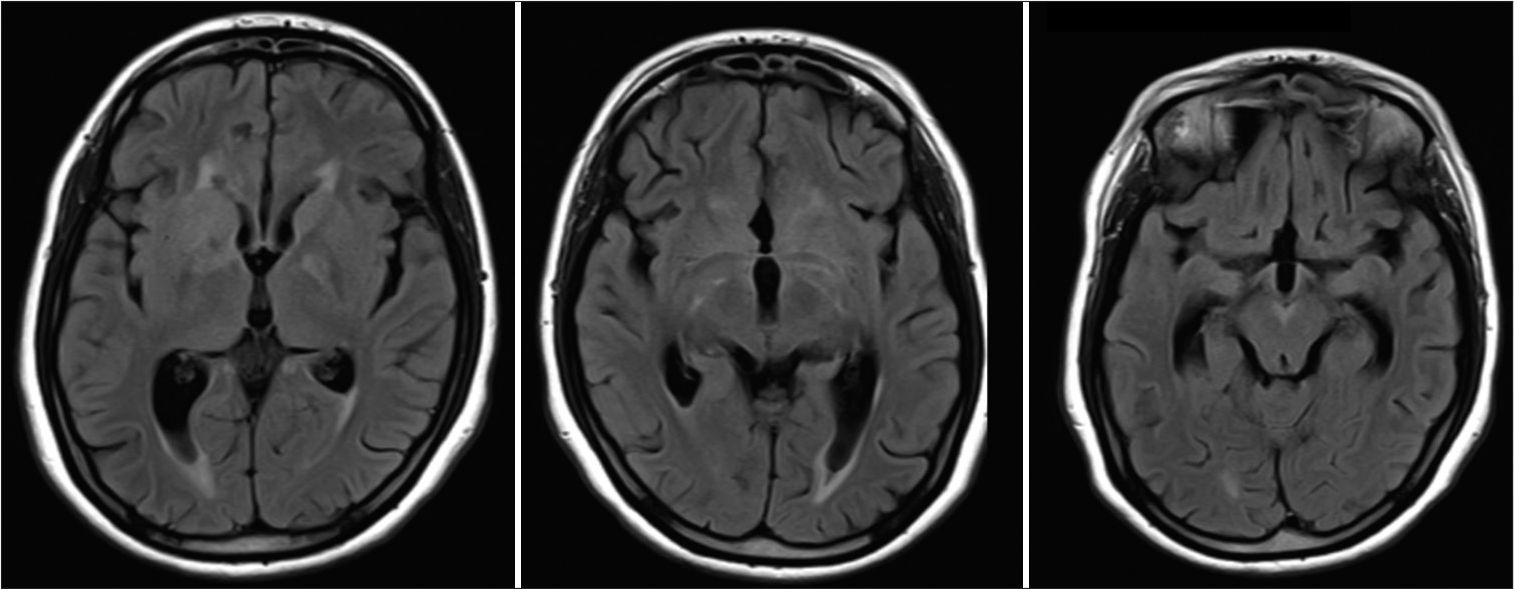
- Fluid-attenuated inversion recovery images 2 weeks post-treatment showing resolution of the previous changes.
DISCUSSION
Bilateral early vision loss with very fulminant course and early optic atrophy, clinical and laboratory evidence of meningeal involvement, sensorineural hearing loss, facial weakness, features of root involvement, and arthritis were important clinical features in this patient.
CNS involvement with vision and hearing loss may be seen in several disorders [Table 1]. However, this list may be further narrowed down as per associated features and infective/ inflammatory etiology was most appropriate possibility in present case. Despite an extensive work-up, we could not find any evidence of inflammatory disease in this patient. CNS tuberculosis, a common disease to be considered in India, may have similar presentation but a fulminant vision loss in 2 days of onset, no other clinical or laboratory evidence of tuberculosis and worsening of symptoms despite appropriate treatment were against the diagnosis. Widespread involvement, characteristic MRI features, positive serology, and response to treatment for borreliosis confirmed the diagnosis of LNB. Radicular pain and arthritis has been reported in variable frequency in American and European series.[1] Recurrent seizure, a rare finding in LNB (reported in 0.5 % cases), was another rare finding in present case. Serology is used for diagnosis of LNB, however, due to complex antigenic property, a second confirmatory test by immunoblot or Western blot is recommended in all positive or equivocal cases.[2] We could not do western blot due to its unavailability at our place. However, characteristic MRI changes and response to specific treatment in the present case were indicative of correct diagnosis of LNB in present case.
| Disease category | Possible diagnosis | Associated clinical features | |
|---|---|---|---|
| Infection | Tuberculosis and other subacute/chronic infections (fungal) leading to pachymeningitis | Constitutional symptoms, fever, features of raised intracranial pressure, papilledema, features of meningitis | |
| Lyme’s disease | Endemic areas, meningitis, painful radiculoneuritis, and cranial neuropathy | ||
| Syphilis | Miotic pupils with light-near dissociation (i.e., Argyll Robertson pupils), other ocular manifestations including uveitis, chorioretinitis, neuroretinitis, retinal vasculitis, and optic neuropathy, acute or subacute sensorineural hearing loss | ||
| Listeriosis | Rhombencephalitis, may cause sensorineural hearing loss, vertigo, facial palsy and rarely, endophthalmitis leading to profound vision loss | ||
| Autoimmune/Inflammatory/infiltrative | Cogan Syndrome | Acute to subacute visual abnormality due to Interstitial keratitis with Meniere-such as attacks of hearing Loss, tinnitus, and vertigo; rarely systemic vasculitis |
|
| Susac syndrome | Inflammatory microvasculopathy; triad of encephalopathy, branch retinal artery occlusions, and vascular hearing loss; infarctions within the body of corpus callosum. | ||
| Vogt-Koyanagi-Harada syndrome | Acute to chronic/recurrent course. Systemic granulomatous autoimmune disease targeting melanocyte-rich tissues, such as the eye (uveitis), inner ear (tinnitus), skin and hair, meninges (neck stiffness, cranial nerve or central nervous system symptoms or cerebrospinal fluid pleocytosis) | ||
| Giant cell arteritis | Vision loss, stroke, occasional acute symptomatic hearing loss which may precede the visual symptoms | ||
| Amyloidosis; Sarcoidosis; Superficial siderosis; Pachymeningitis (including IgG4–related disease); neurolymphomatosis; Systemic vasculitis | Disease specific associated features | ||
| Metabolic | Refsum disease | ||
| Fabry disease | |||
| Mitochondrial | MELAS and other mitochondrial syndromes | Maternal Inheritance, myopathy, neuropathy, ataxia, and retinitis Pigmentosa, encephalopathy, increase lactate |
|
| Inherited Disorders | Usher syndrome | Retinitis pigmentosa, profound sensorineural hearing loss, imbalance. | |
| Wolfram syndrome (DIDMOADS) | Childhood onset, progressive optic atrophy, truncal ataxia, startle myoclonus, reduced limb reflexes, horizontal nystagmus, dysarthria, central apneas, loss of taste and smell, and hemiparesis | ||
Ocular involvement, although uncommon, can be seen in all the three stages of LNB specially in endemic zone. Conjunctivitis, keratitis, and other inflammatory syndrome involving some or all parts of eye and optic neuritis are reported in Lyme’s disease. Bilateral papilledema is observed in a minority of cases with meningitis possibly due to increased intracranial pressure. Isolated papillitis, as in present case, due to Lyme’s disease is difficult to diagnose, but associated arthritis, radicular pain, and other constitutional symptoms may be few useful clues.
Most reported MR imaging features in neuroborreliosis are T2/FLAIR changes in subcortical white matter and basal ganglia, cortical infarcts, contrast enhancement of cranial nerves, meningeal enhancement in brain, and spinal cord and myelitis, especially in cervical region.[3-5] Enhancement patterns ranged from no-enhancement to nodular or diffusely extensive contrast-enhancement.[6] All of those features were seen in our patient, as has been already described. Furthermore, these features and contrast enhancement markedly reduced after treatment [Figure 4]. Characteristic contrast enhancement in “M” pattern, an arc-shaped edema of the lateral pontine nuclei, and pontocerebellar fibers in MRI was reported by Pfefferkorn in a series of five patients.[7] Similar pattern of rounded “M” was seen in our case in the pons as well as anterior midbrain. Other characteristic patterns include mesencephalic edema, sparing the red nucleus, substantia nigra, and the long pathways.[7] Of note, first image did not show these changes and also repeat imaging after therapy clearly indicated a marked reduction in the size of this lesion.
CONCLUSION
It would be helpful for clinicians to know these radiological features of neuroborreliosis, including the contrast enhancement along multiple cranial nerves and pattern resembling rounded “M” in brainstem. These features would be especially helpful in unusual presentations like in our case, in resource limiting settings where extensive investigations are seldom possible due to economic constraints.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Lyme neuroborreliosis: Manifestations of a rapidly emerging zoonosis. AJNR Am J Neuroradiol. 2009;30:1079-87.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 1995;44:590-1.
- [Google Scholar]
- Neuro-lyme disease: MR imaging findings. Radiology. 2009;253:167-73.
- [CrossRef] [PubMed] [Google Scholar]
- Biopsy-confirmed CNS Lyme disease: MR appearance at 1.5 T. AJNR Am J Neuroradiol. 1990;11:482-4.
- [Google Scholar]
- Common and uncommon neurological manifestations of neuroborreliosis leading to hospitalization. BMC Infect Dis. 2017;17:90.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging in Lyme neuroborreliosis. Insights Imaging. 2018;9:833-44.
- [CrossRef] [PubMed] [Google Scholar]
- Brainstem encephalitis in neuroborreliosis: Typical clinical course and distinct MRI findings. J Neurol. 2021;268:502-5.
- [CrossRef] [PubMed] [Google Scholar]






